WHat charges to the following subatomic particles have:
PROTONS=?
NEUTRONS=?
ELECTRONS=?
PROTONS = +
NEUTRONS= NO CHARGE/NEUTRAL
ELECTRONS=NEGATIVE
A sample of water was collected from a local stream. Data from a water quality sample produced the following results:
The water was visibly clear
The water contains dissolved salts
Based on this data, how should this water sample be classified?
Element
Compound
Homogeneous mixture
Heterogeneous mixture
Homogeneous mixture
You’re helping to sort elements for a project, and you need to find the element in Period 5, Group 2. Which element should you select?
Strontium (Sr)
During a fireworks display, colorful explosions light up the sky, releasing heat and sound. Are the changes occurring in the fireworks physical or chemical?
A. Physical Changes
B. Chemical Changes
C. Both Physical and Chemical Changes
D. Neither Physical or Chemical Changes
Chemical Changes
Identify the ELEMENTS in each equation: (Example: S= Sulfur)
2H2+O2→2H2O
2Na+O2→Na2O
N2+3H2→2NH3
1. HYDROGEN, OXYGEN
2. SODIUM, OXYGEN
3. NITROGEN, HYDROGEN
Sarah is studying a model of an atom and notices that one particle is much smaller than the others and moves around outside the center. Which subatomic particle is she observing?
a) Proton
b) Neutron
c) Electron
d) Nucleus
Electron
A sample of metal was tested in a laboratory. It was found that the piece of metal contained atoms that all had 79 protons. How should this sample be classified?
An Element
A Compound
A mixture
A solution
An Element
During an experiment, you encounter an element that is brittle, does not conduct electricity, and is often a gas at room temperature. What type of element is this: metal or nonmetal?
A. Metal
B. Nonmetal
C. Metalloid
D. Alkali Metal
Nonmetal
A large piece of zinc was broken into pieces and one piece was placed in a test tube with an unknown liquid. Bubbles formed and the zinc changed from silver to bronze. What most likely occurred?
HINT: TWO CHANGES
The zinc underwent a physical change when it was broken and a chemical change in the liquid
Fill in the blank for the following:
1. ______________: The small number after an element that shows how many atoms of that element are in one molecule.
2. _____________: A number in front of an element or compound that tells you how many molecules of that substance are involved in the reaction
coefficient
You are given an atom with 15 protons, 16 neutrons, and 15 electrons to model. In this atom, where are the protons, neutrons, and electrons located, and what are their charges?
Protons and neutrons are in the nucleus, electrons are in the orbitals. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge.
If a liquid separates after mixing, how should it be classified?
Element
Compound
Homogeneous mixture
Heterogeneous mixture
Heterogeneous mixture
In a workshop, you’re choosing an element that can be easily stretched into wires without breaking and is highly malleable. Which element most likely has these characteristics?
A. Fluorine (F)
B. Phosphorus (P)
C. Helium (He)
D. Copper (Cu)
Copper (Cu)
During a fireworks display, colorful explosions light up the sky, releasing heat and sound. Would you classify the reaction in fireworks as endothermic or exothermic?
A. Endothermic; energy is absorbed in the form of heat
B. Exothermic; energy is released in the form of heat
C. Endothermic; energy is released in the form of heat
D. Exothermic; energy is absorbed in the form of heat
B. Exothermic; energy is released in the form of heat
Count the number of atoms on both the reactants and products side.
2Na+O2→Na2O
IS THIS EQUATION BALANCED OR UNBALANCED?
Product: REACTANT=
Na= 2 Na= 2
O= 1 O= 2
UNBALANCED
IDENTIFY THE ELEMENT USING THE IMAGE:
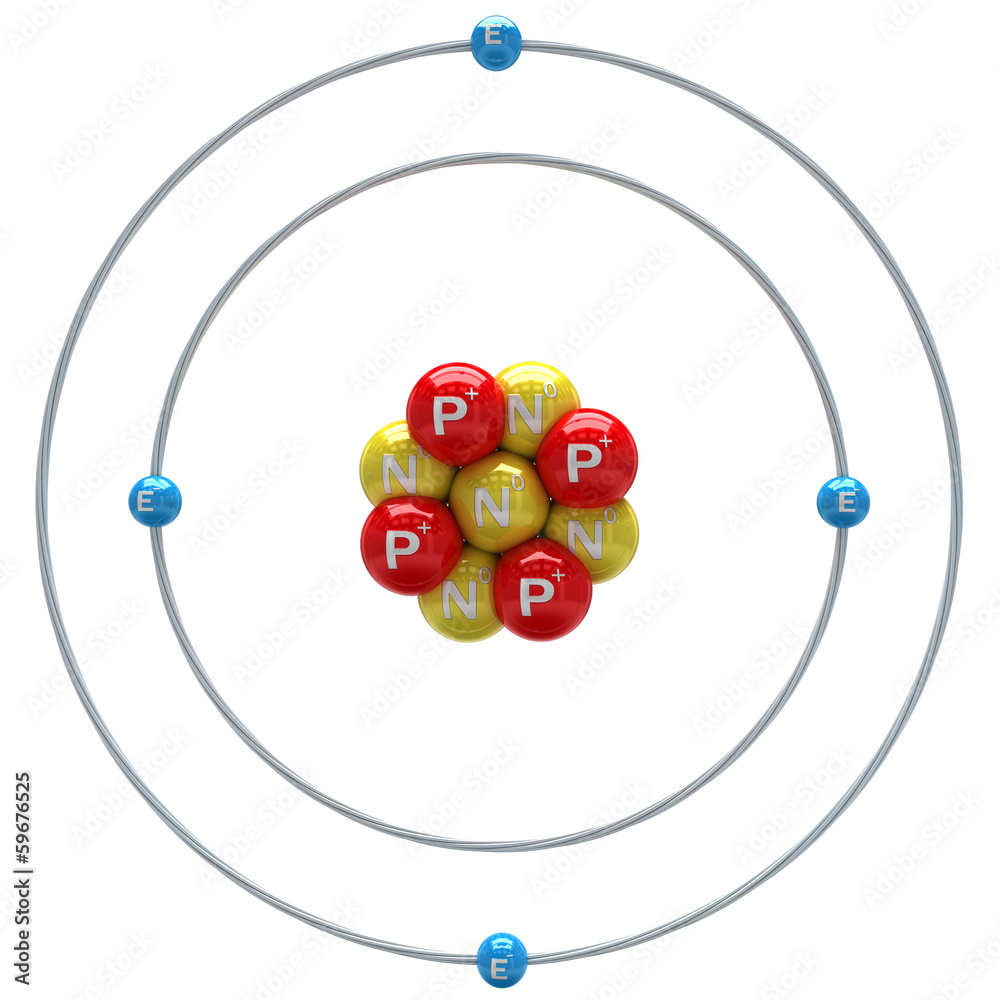
Be
An unknown powered substance contains molecules of silver (Ag), lead (Pb), and iron (Fe). All of the molecules are identical when examined under a microscope. How should this substance be classified?
Element
Compound
Homogeneous mixture
Heterogeneous mixture
Compound
Which element is most likely to have the same properties as Magnesium (Mg)?
A. Sodium (Na)
B. Silicon (Si)
C. Calcium (Ca)
D. Iron (Fe)
Calcium (Ca)
Two clear solutions are mixed together, and a solid substance suddenly forms and settles at the bottom of the container. What evidence indicates that a chemical change has occurred?
A. The temperature of the mixture increased.
B. A gas was released from the mixture.
C. The mixture remained clear and unchanged.
D. A solid precipitate formed from the reaction.
D. A solid precipitate formed from the reaction.
In a lab, students combine calcium (Ca) and chlorine gas (Cl₂) to make calcium chloride (CaCl₂). They need to find the correct number to balance the equation.
Ca + Cl₂ → __CaCl₂
What number should go in front of CaCl₂ to balance the equation?
A. 1
B. 2
C. 3
D. 4
1
You are working in a science lab where you've been given a mystery element to study. The lab notes describe this element as having 11 protons, 12 neutrons, and 11 electrons. Based on this information, draw a model of the atom for this mystery element. Label the protons, neutrons, and electrons.
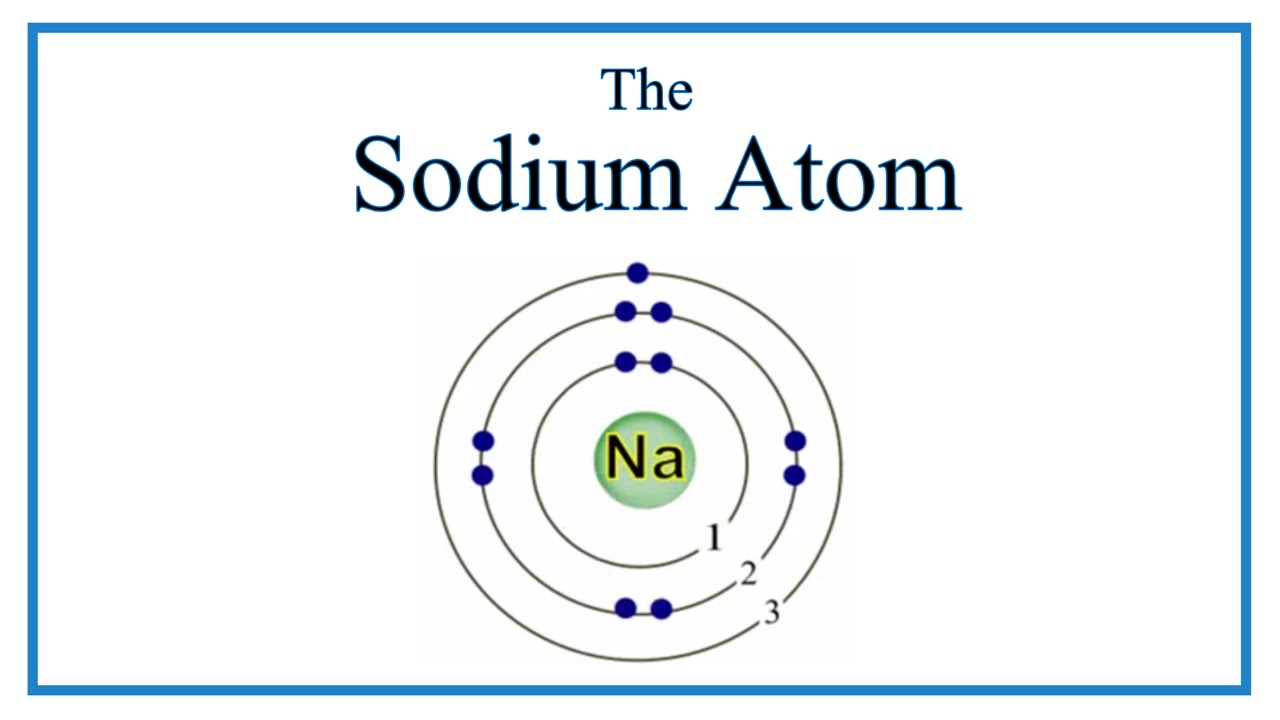
DISTINGUISH THESE IMAGES (MIXTURE, COMPOUND OR ELEMENT)
1. COMPOUND
2. ELEMENT
3. COMPOUND
4. ELEMENT
TWO PARTS:
1. WHAT GROUP IS NON-REACTIVE?
2. Potassium (K) is most likely to form a chemical bond with what other element?
A. Chlorine (Cl)
B. Calcium (Ca)
C. Sodium (Na)
D. Zinc (Zn)
1. GROUP 18
2. Chlorine (Cl)
Which statement best compares the following scenarios?
-A bike is left outside for two weeks and a layer of rust forms on the metal.
-A pot of water is left on the stove until the water is gone
A. Both scenarios describe physical changes
B. Both scenarios describe chemical changes
C. Scenario 1 describes a physical change, while scenario 2 describes a chemical change
D. Scenario 1 describes a chemical change, while scenario 2 describes a physical change
D. Scenario 1 describes a chemical change, while scenario 2 describes a physical change
In a closed system, a chemical reaction between methane (CH₄) and oxygen (O₂) produces carbon dioxide (CO₂) and water (H₂O).
CH₄ + 2O₂ → CO₂ + 2H₂O
According to the law of conservation of mass, what happens to the mass after the reaction?
A. The mass increases because more molecules are produced.
B. The mass decreases because the reactants are consumed.
C. The mass remains constant because matter is neither created nor destroyed.
D. The mass changes due to energy being absorbed or released.
C. matter is neither created nor destroyed.