Define a covalent bond.
A chemical bond that involves the sharing of electrons to form electron pairs between atoms.
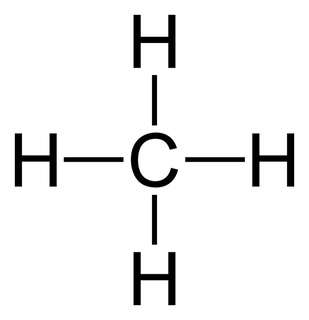
Draw the Lewis structure for CH4
The central carbon atom is surrounded by four single bonds, each connected to a hydrogen atom.
State the VSEPR theory.
VSEPR theory states that electron pairs around a central atom repel each other and arrange themselves to minimise repulsion.
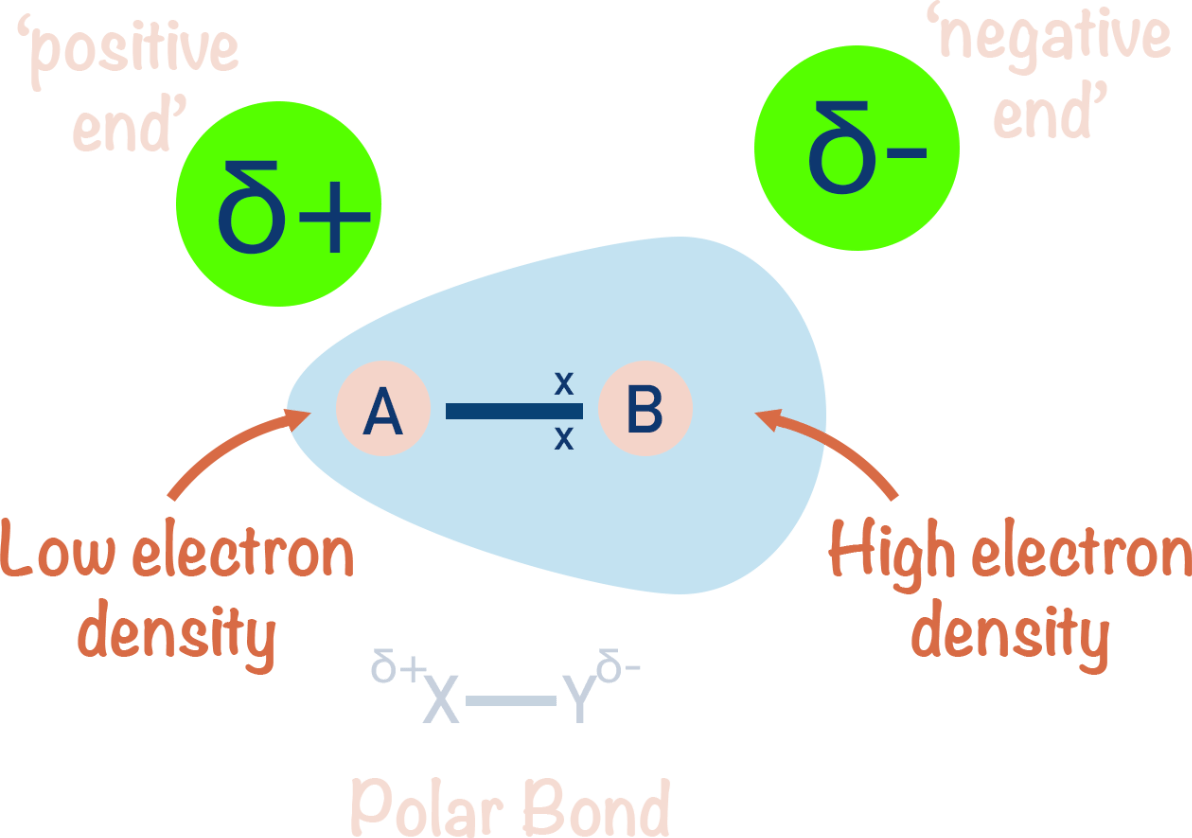
Define electronegativity.
Electronegativity is the ability of an atom to attract shared electrons in a covalent bond.
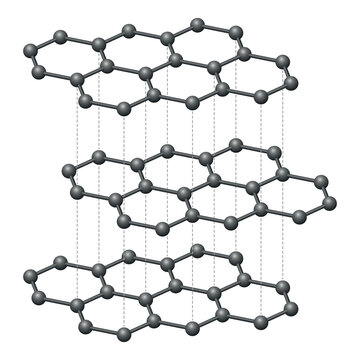
Define an allotrope.
An allotrope is a different structural form of the same element in the same physical state.
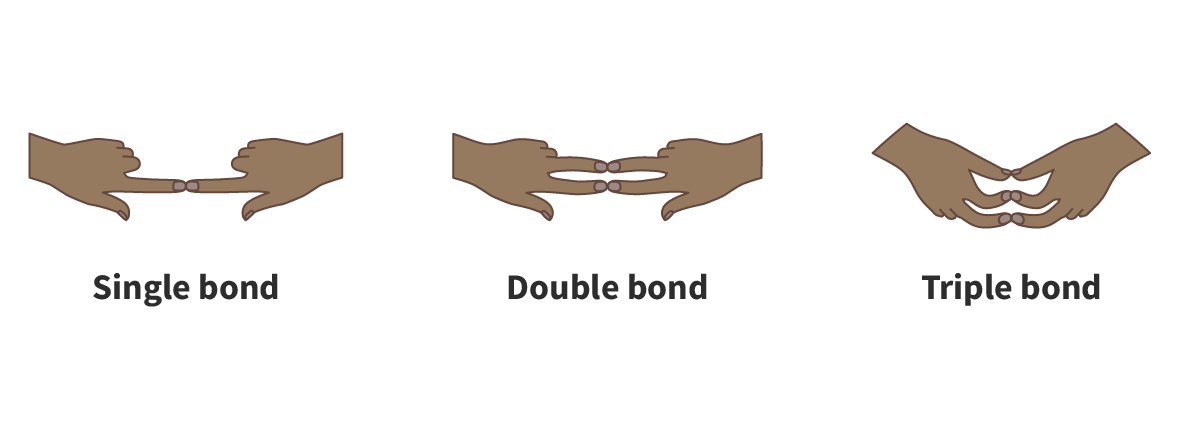
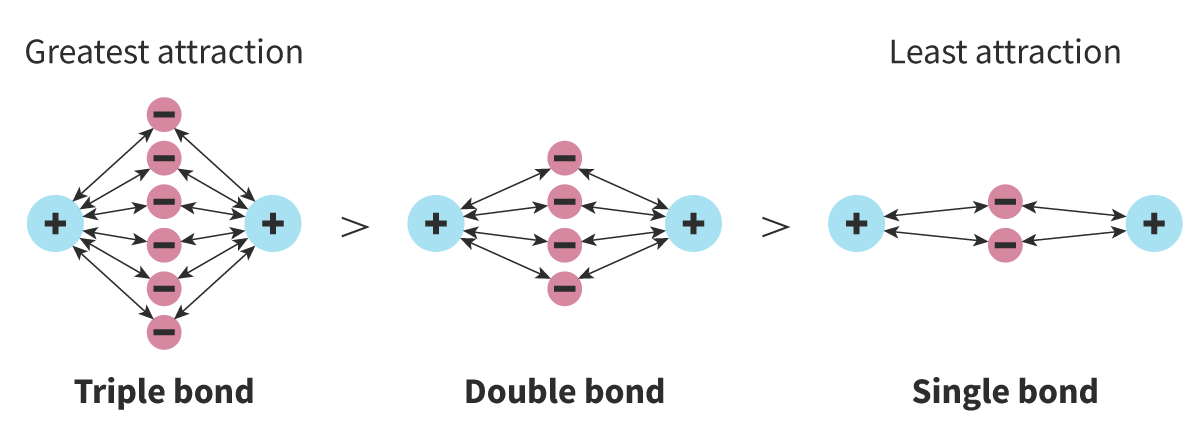
Describe the relationship between bond strength and bond length.
Shorter bonds are stronger than longer bonds. Double bonds are shorter and stronger than single bonds, and triple bonds are shorter and stronger than double bonds.
Deduce the Lewis structure for CO2.
The central carbon atom forms two double bonds, each connected to an oxygen atom.
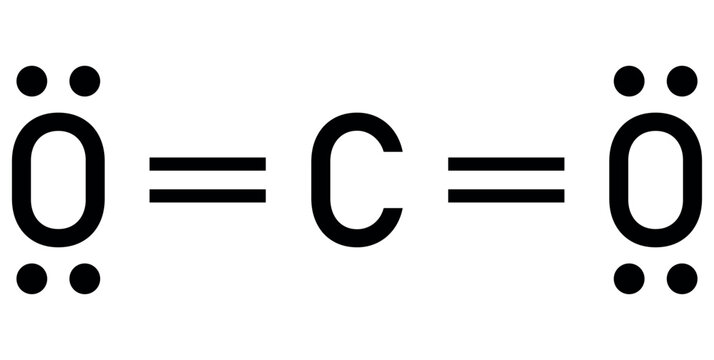
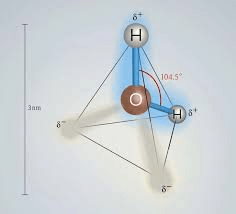
Predict the geometry of H2O.
Bent (V-shaped) geometry with a bond angle of approximately 104.5°.
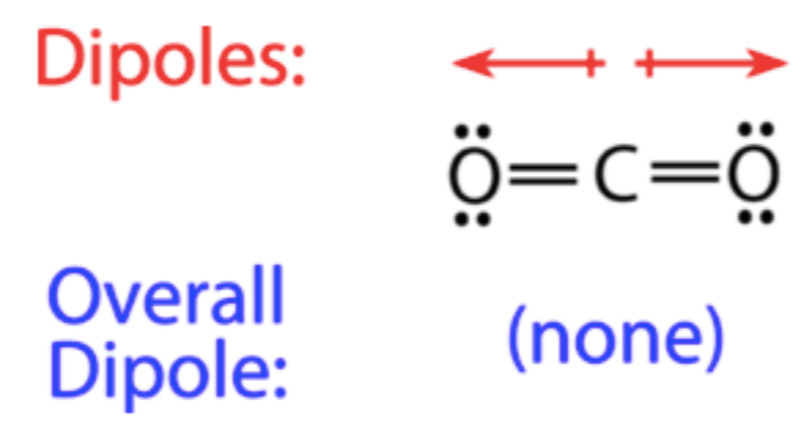
Deduce if CO2 is polar or nonpolar.
CO2 is nonpolar because the polar bonds are symmetrically arranged, canceling the dipole moments.
Describe the structure of diamond.
Diamond consists of carbon atoms covalently bonded in a tetrahedral lattice.

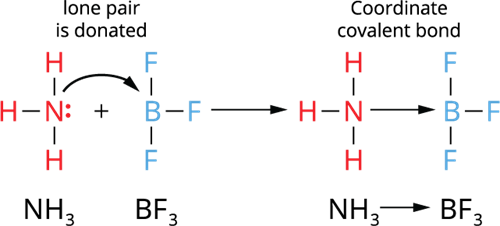
Explain the concept of a coordination bond.
A coordination bond is a covalent bond where both electrons in the shared pair come from one of the bonding atoms.
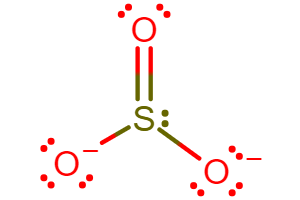
Predict the Lewis structure for SO32−.
The central sulfur atom is bonded to three oxygen atoms: two with single bonds and one with a double bond, with lone pairs and a 2- charge distributed across the structure.
Compare the geometries of SF6 and NH3.
SF6 has an octahedral geometry, while NH3 has a trigonal pyramidal geometry due to the lone pair.
Describe how molecular shape influences dipole moments.
Asymmetrical shapes result in a net dipole moment, while symmetrical shapes often result in dipole moments canceling out.
Compare the structures and properties of diamond and graphite.
Diamond has a 3D tetrahedral structure, making it hard and non-conductive. Graphite has layered hexagonal sheets, making it soft and conductive.

Compare double and triple bonds in terms of length and strength.
Double bonds are shorter and stronger than single bonds, while triple bonds are even shorter and stronger than double bonds.
Explain why some molecules have incomplete octets.
Molecules like BF3 have an incomplete octet because the central atom, boron, has only six valence electrons.
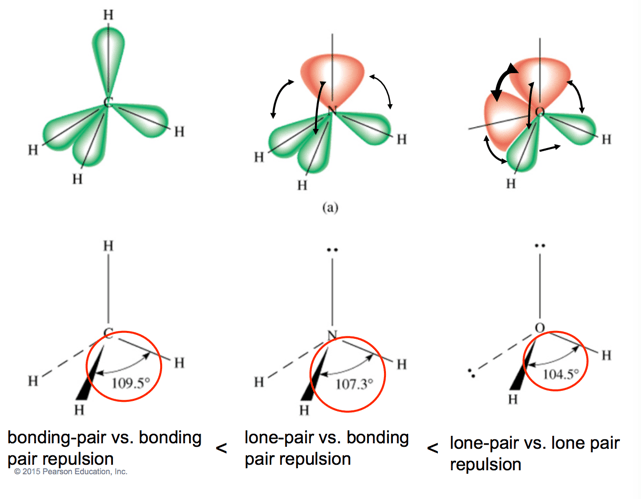
Explain how non-bonding pairs affect bond angles.
Non-bonding pairs create greater repulsion than bonding pairs, reducing bond angles in the molecule.
Explain why CHCl3 is polar.
It is polar because its tetrahedral shape creates an asymmetrical distribution of dipoles.
Explain why silicon dioxide has high melting points.
Silicon dioxide has a covalent network structure with strong covalent bonds throughout, requiring high energy to break.

Identify the significance of covalent bonds in fulfilling the octet rule.
Covalent bonds allow atoms to share electrons, enabling them to achieve a full valence shell of eight electrons.
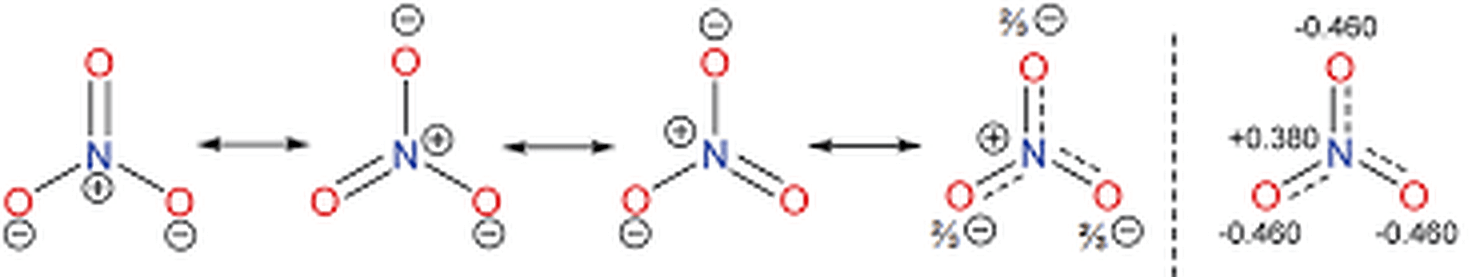
Determine the formal charges in NO3−.
One oxygen atom has a formal charge of -1, and the others are 0. The nitrogen atom also has a formal charge of 0.
Name of this this cat?
Chemistry Cat / ChemCat
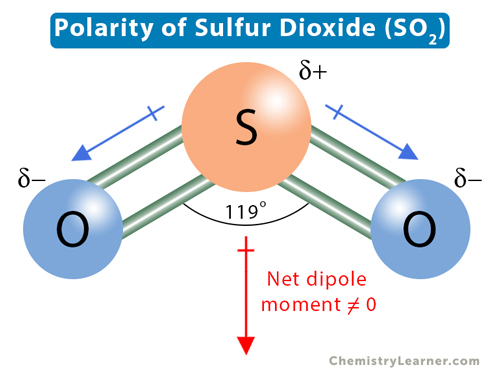
Predict the net dipole moment of SO2.
SO2 has a net dipole moment because it has a bent shape, and the bond dipoles do not cancel out.
Discuss the electrical conductivity of graphite.
Graphite conducts electricity because it has delocalised electrons within its layers that can move freely.