The ONE part of the experiment that can be changed is the...
INDEPENDENT variable
What is the metric unit used to measure the amount of matter in an object?
GRAMS is used to measure mass.
An object is heated.
a. What happens to the particle motion?
b. Are the particles absorbing or releasing energy?
a. Particle motion increases, the particles move faster
b. Particles are absorbing energy
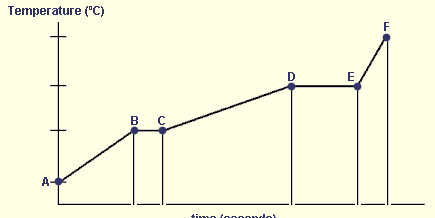
Between which points would evaporation occur?
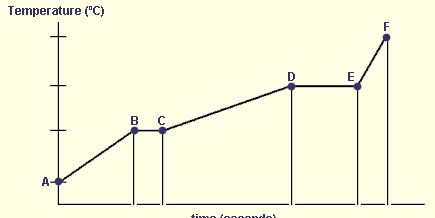
D to E
Waves transfer _____.
Energy
Identify the location of each of the 2 subatomic particles.
protons- nucleus
neutrons- nucleus
electrons- energy shells around nucleus (or electron cloud)
What is the part of the experiment that does not receive the change and is used for comparison?
the CONTROL Group
What is the formula for density?
density = (mass)/(volume)
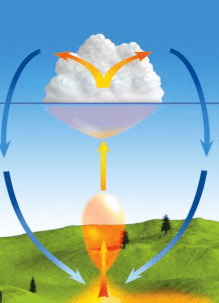
What type of heat transfer is involved in cloud formation?
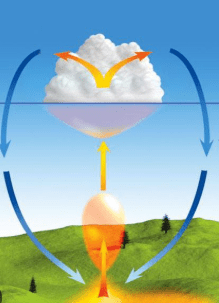
Convection
Electricity is the flow of ____________.
Electrons
The height or the wave from the rest to crest.
How many hydrogen atoms are in 3C6H12O6?
36
Label each statement as observation or inference in the photo.

a. There is one dog.
b. The dog ate the pillow.
c. The couch is grey.
a. There is one dog.- OBSERVATION
b. The dog ate the pillow.- INFERENCE
c. The couch is grey.- OBSERVATION
What is the volume of the button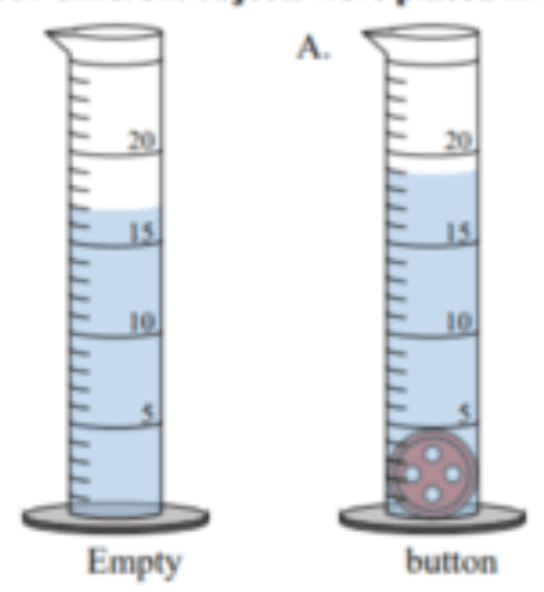 ?
?
2 mL (19 mL - 17 mL)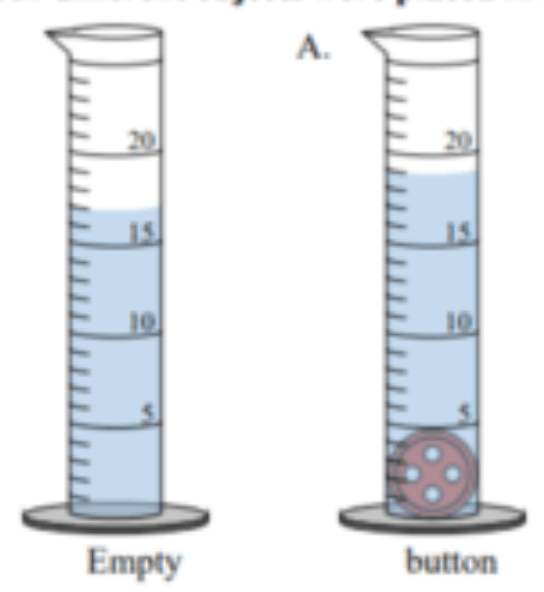
What happens to the total amount of energy as a cart moves down a roller coaster?
total amount of energy remains the same
(Law of Conservation of Energy)
In a parallel circuit what happens to the other lights if one of the bulbs goes out?
the other bulbs will remain lit.
Identify the different behavior of lights pictured.
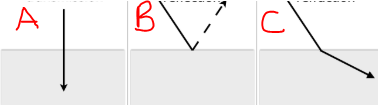
a. Transmission
b. Reflection
C. Refraction
Identify the following as a chemical change or physical change.
a. metal rusting
b. burning fuel
d. dissolving salt in water
a. metal rusting - CHEMICAL
b. burning fuel- CHEMICAL
d. dissolving salt in water- PHYSICAL
How does the temperature of a tennis ball affect the height of its bounce?
What is a correctly written hypothesis for this problem?
a. If I heat up a tennis ball then it will be hot.
b. If I freeze a tennis ball then it will not bounce as high.
c. Frozen tennis balls will not bounce very high.
b. If I freeze a tennis ball then it will not bounce as high.
What is the mass?
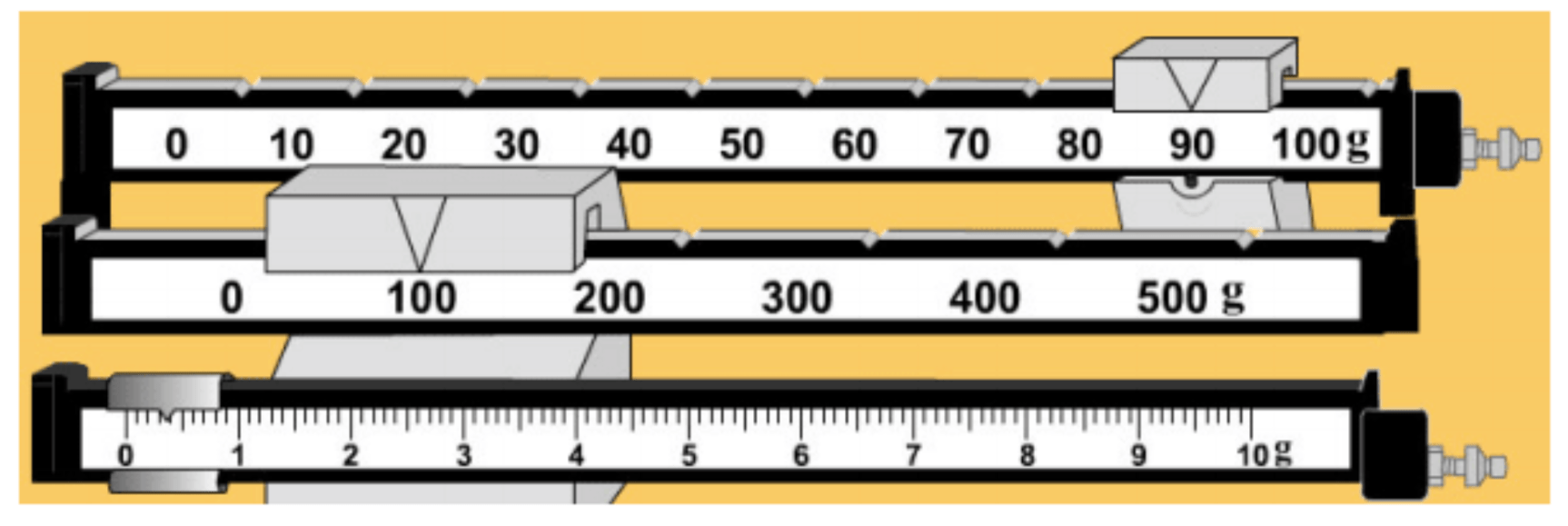
190.4 grams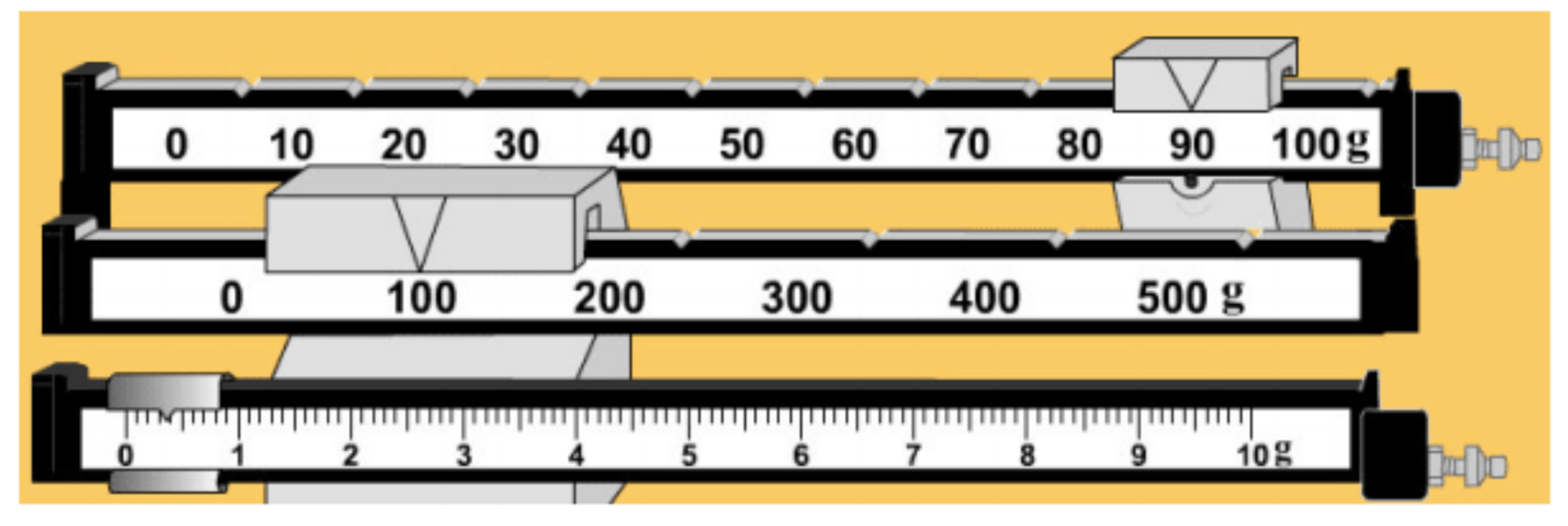
You touch a stove and burn your hand. Describe the flow of energy (____ to ___)
from the HOT STOVE to the COOLER HAND
Identify the type of circuits below.
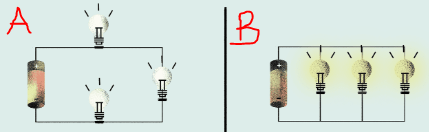
a. Series
b. Parallel
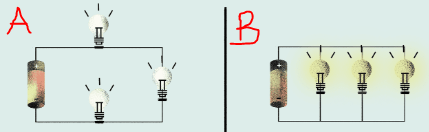
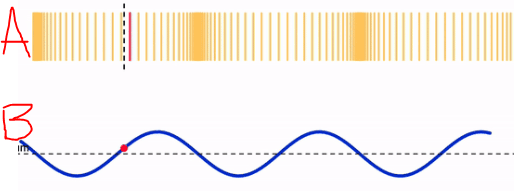
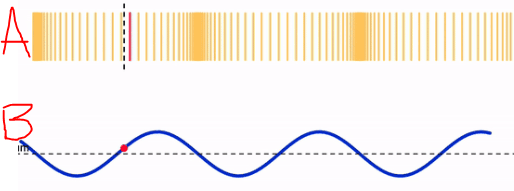 Identify the each type of wave pictured.
Identify the each type of wave pictured.
A. Longitudinal
B. Transverse
Balance this equation:
H2 + Cl2 --> HCl
H2 + Cl2 --> 2HCl
How does the temperature of a tennis ball affect the height of its bounce?
Identify the INDEPENDENT and DEPENDENT variable in this experiment.
IV= temperature of tennis ball
DV= height of bounce
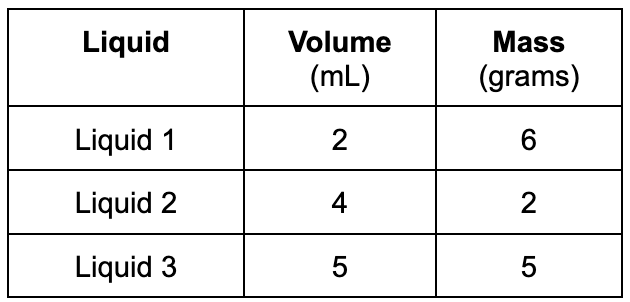
What would be the correct order (from TOP to BOTTOM) if the liquids were poured into a glass cylinder?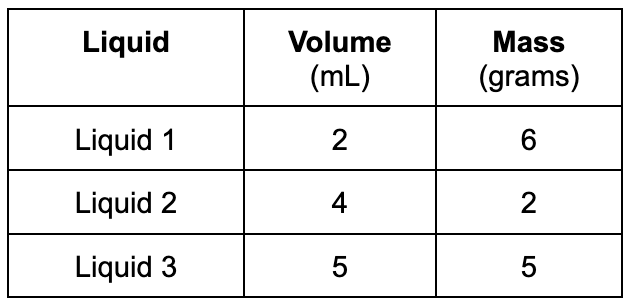
Top= Liquid 2
Middle= Liquid 3
Bottom= Liquid 1
Do the dance for heat transfer
conduction, convection, radiation
(watch teacher)
Identify the electric conductors:
rubber ball, paperclip, coin, dollar bill, glass, metal fork
paperclip, coin, metal fork
Given the electromagnetic spectrum, which type of wave has the lowest energy? highest energy?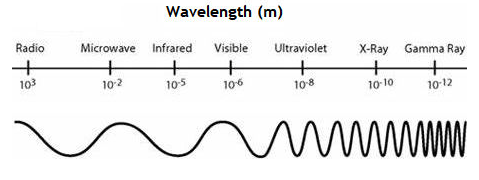
Lowest- Radio
Highest- Gamma
How many valence electrons does oxygen contain?

6
