What is the charge of a proton?
Positive
What is the maximum number of electrons that can fit in the first shell?
2 electrons
What is a valence electron?
Electrons that are on the outermost shell/layer of an atom
Identify the number of:
Hydrigen and Oxygen
2H₂ + O₂
H: 4
O: 2
Name the Movie:
Inception
What is the charge of a neutron
No Charge (Neutral)
Draw the Bohr Model for Lithium
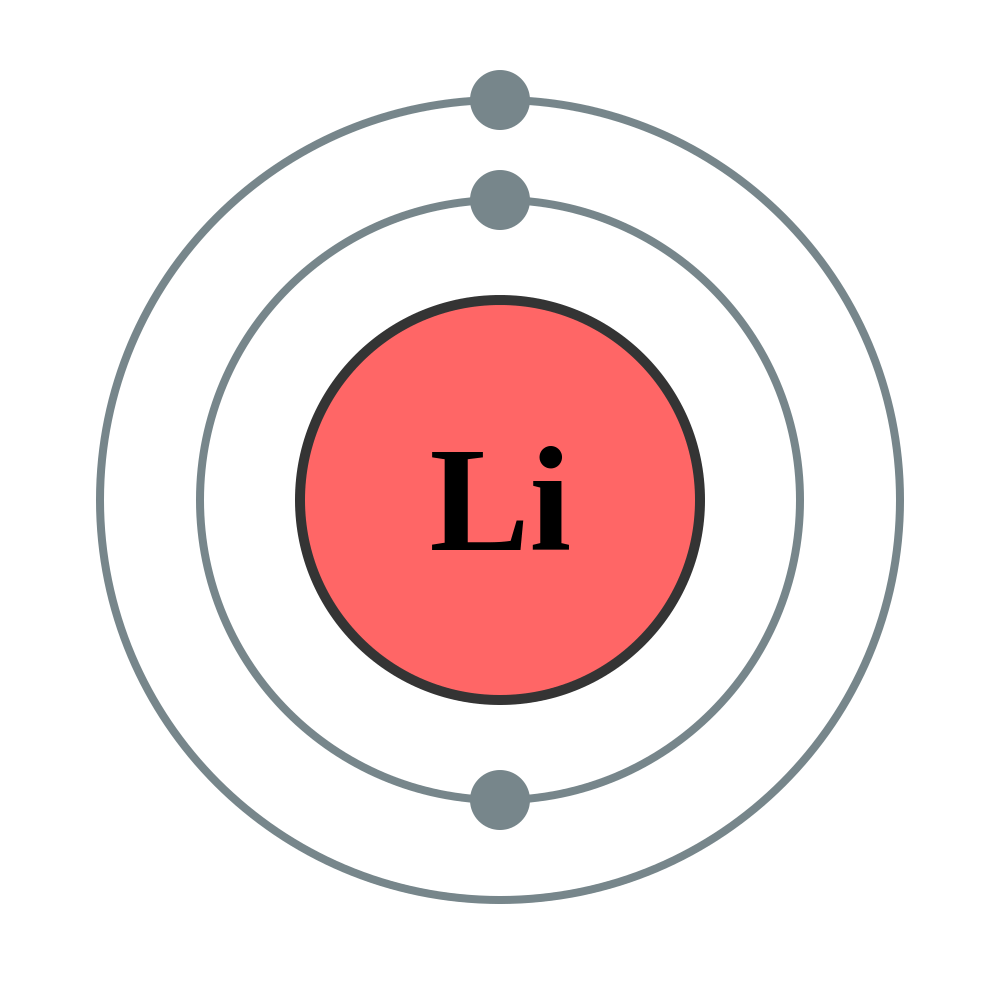
How many valence electrons does Socium (Na) have?
1 Valence electron
Identify the number of
Ca, O & H
Ca(OH)₂
Ca: 1
O: 2
H: 2
Name the movie:
Matrix Reloaded
What is the negative subatomic Particle called?
Electron
Draw the Bohr Model for Nitrogen
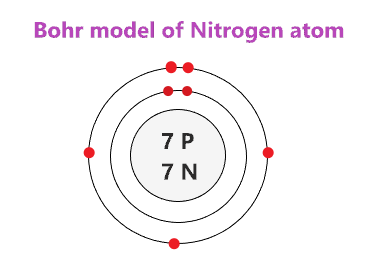
How many valence electrons does Magnesium (Mg) have?
2 Valence Electrons
Balanced or Unbalanced?
H₂ + O₂ → H₂O
Unbalanced
Name the Song and Artist
Umbrella:
Rihanna
If a normal element has 11 protons how many electrons will it have?
11 Eelctrons
What element does this Bohr Model show
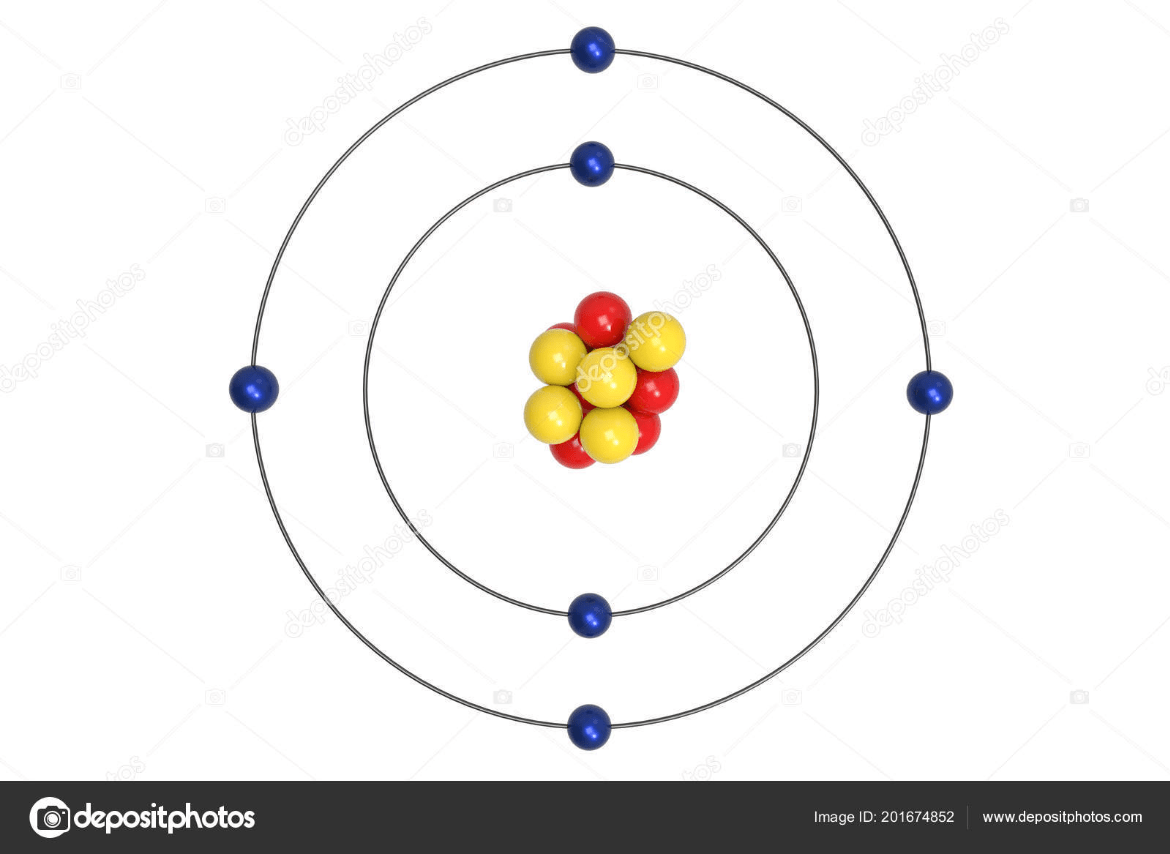
Carbon
All atoms want a full outer shell (8 for the outershells and 2 for the inner shell) to become stable.
How many electrons does lithum have to loose/gain to become stable?
Lose 1
Balanced or Unbalanced
2Na + Cl₂ → 2NaCl
Balanced
Which language has the most native speakers in the world?
Mandarin chinese
What subatomic particles have little or no mass?
Electrons
Identify the element: 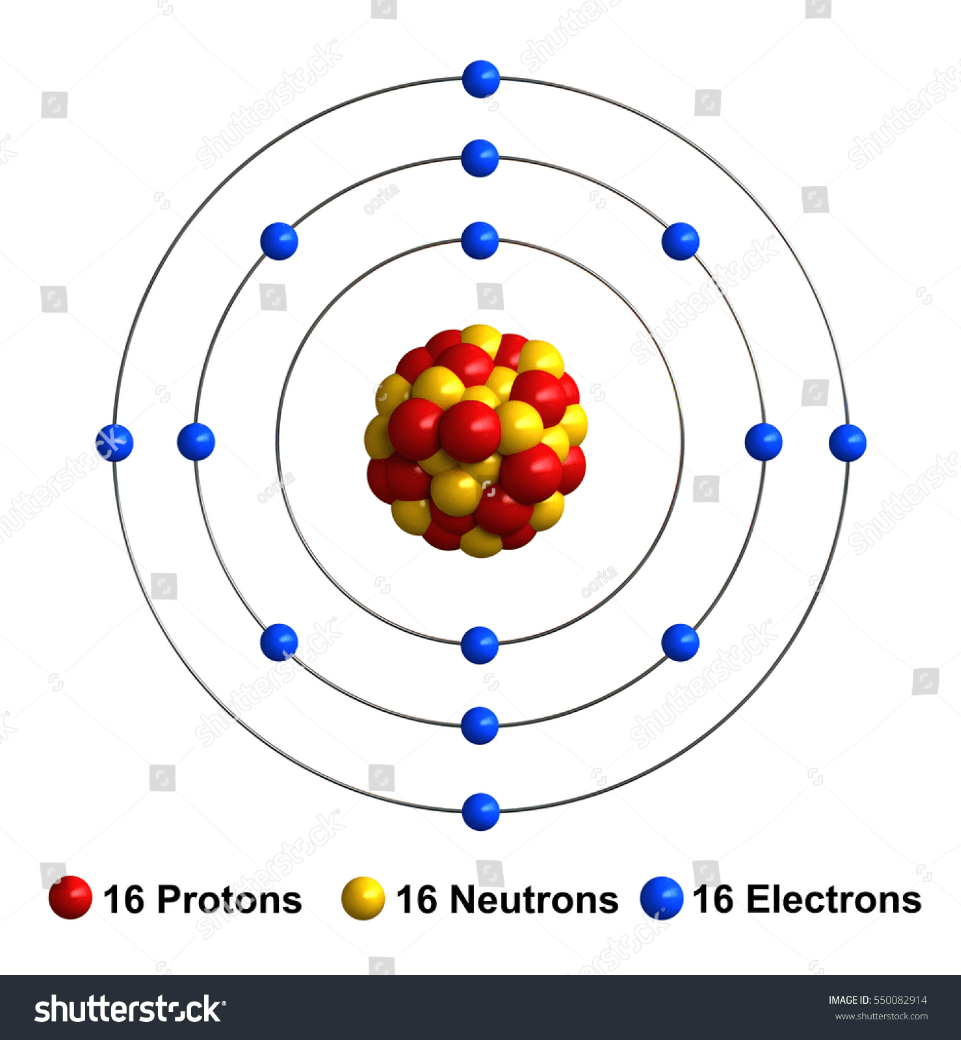
Sulfur
How many electrons does Nitrogen have to loose/gain to become stable?
Gain 3 electrons
Balance the equation:
Fe + O₂ → Fe₂O₃
4Fe + 3O₂ → 2Fe₂O₃
What is the rarest blood type in the world?
AB Negative