Scientist that is credited with development of the Atomic Theory.
John Dalton
Boron is considered one of these type of elements because it lies on the staircase on the Periodic Table.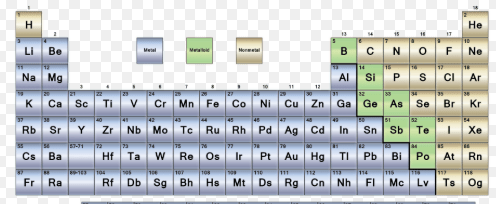
Metalloid
A metal like sodium can lose an electron to bind with chlorine, a non metal to form this type of bond
Ionic
How many sodium atoms are in 4 molecules of table salt?
NaCl
4
In which state of matter (Solid, liquid, gas) do particles have the least energy?
Solid
JJ Thompson used cathode ray tubes studying the atom to discover these negative particles existed.
Electrons
Group 18 (Noble gases) are nonreactive because they have full this?
Outer or valence shells
Two nonmetals like Carbon and Oxygen will bond by sharing electrons in this type of bond:
Covalent
In the formula C4H10 how many Carbon atoms are there?
4
In the equation below what is the product?
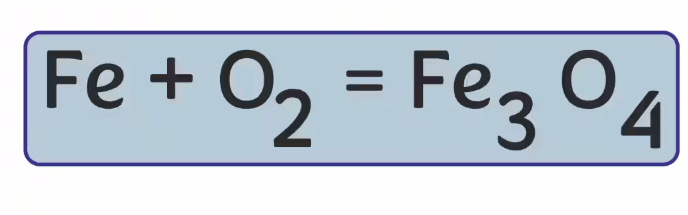
Fe3O4
Rutherfords Gold foil experiment led to discovery of this part of the atom that contains both positive and neutrally charged particles.
Nucleus
Elements in the same group react similarly because they have the same number of these:
Outer shell or valance shell electrons
Atoms with the same number of protons but different numbers of these are called isotopes
Neutrons
How many total atoms are in a molecule of
C6H12O6
24
An unknown element appears shiny, conducts heat well and is malleable. This element is most likely a _________.
Metal
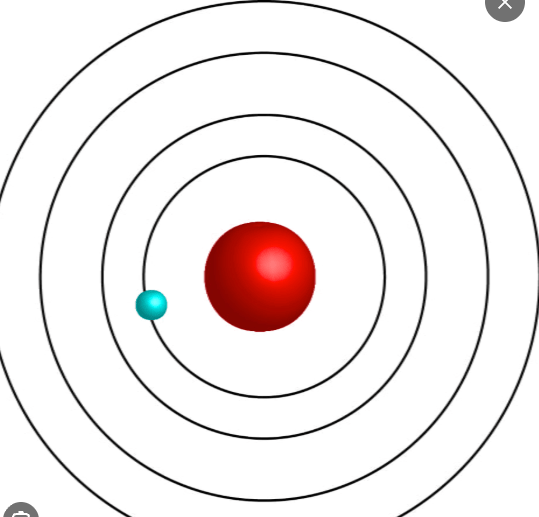 Also known as the planetary model this model was developed by this man.
Also known as the planetary model this model was developed by this man.
Neils Bohr
An atom with 40 protons and 37 neutrons has an atomic number of what?
40
If a Calcium ion is formed by Calcium giving up to electrons what is the charge of the Calcium ion?
+2
 Formation of this _____________ like the yellow substance above is an indicator of a chemical reaction
Formation of this _____________ like the yellow substance above is an indicator of a chemical reaction
Precipitate
In Science, these universal truths that describe a phenomena are called these.
Scientific law
Ancient Greek philosopher who thought atoms were solid indivisible particles gave the atom its name.
Democritus
The period an atom is in tells us it has this many what?
Energy levels or shells
The difference between Carbon-12 and Carbon-14 is they have different numbers of neutrons. How many neutrons would Carbon-14 have? 
8
What evidence here is an indicator that a chemical reaction is occuring? 
Bubbles, fizzing, gas
John Dalton is credited with development of the Atomic _____________ of Matter which attempts to explain our understanding of atomic structure.
Theory