What is the charge of a Proton?
What is positive
The energy of a photon is related to its
frequency
A subatomic particle that has no charge and that is found in the nucleus of an atom, has mass
neutrons
how many protons are in this atom?

What is 12
This type of radioactive decay happens when a neutron changes into a proton and releases a particle.
What is beta decay?
What is the mass of a proton?
What is 1 amu
What is the correct notation for the sublevel within the first energy level?
1s
What is a neutron
When Uranium-235 undergoes alpha decay, it becomes
What is Thorium-231
The tiny particle that is released during beta decay and has a negative charge.
What is a beta particle (or electron)?
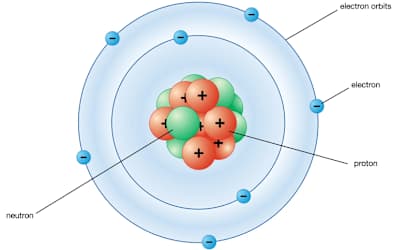
How many protons does this atom have.
What is 7 protons
What is the maximum number of electrons that a single orbital can hold?
2
What charge does a Neutron have?
no charge
Americium- 243 is the daughter nucleus of the alpha decay of what radioactive isotope? (what was it before it decayed?)
Berkelium-247
During beta decay, this part of the atom stays the same while the atomic number changes.
What is the mass number?
What does the number of protons tell you about the atom?
What is the atomic number
How many electrons can the "s" sublevel hold?
2
How do you find the # of Neutrons?
Mass-# of Protons
Ions have different number of
What is neutrons
In beta decay, a neutron turns into a proton, an electron, and this nearly massless particle.
What is a neutrino?
What is 61
According to the Bohr model of the atom, which particles are allowed to exist in any one of a number of energy levels?
electrons
What particles are found inside the nucleus of an atom?
Protons and neutrons
What is the charge of an alpha particle?
What is 0
After beta decay occurs, the atom becomes a different element because this number increases by one.
What is the atomic number?