List each element as a metal or a nonmetal
Carbon
Copper
Carbon - Nonmetal
Copper - Metal
What is a name for CO2?
Carbon Dioxide
What are 4 of the 5 types of reactions?
Synthesis
Decomposition
Single Replacement
Double Replacement
Combustion
What are 2 out of the 3 different levels of saturation in a solution?
Unsaturated
Saturated
Supersaturated
What pH is considered neutral?
7
Name one musical instrument that has strings.
Guitar, violin, cello, piano, harp, etc.
How many protons and neutrons does phosphorus-33 have?
Protons = 15
Neutrons = 18
What would the formula for Ruthenium (II) Chloride be?
RuCl2
Which type of reaction will have 2 or more reactants that combine to make 1 final product?
Synthesis
If a chemical contains the element sodium, will it be soluble or insoluble?
Soluble (Alkali metal/Capital N)
Is the pH of 7.2 acidic, basic, or neutral?
Basic
What does the prefix octo- mean when placed before a word?
8
(octagon, octapus)
Out of all of these elements, which will have the largest atomic radius?
Rb, Mo, Ga
Rb
What would the name of XeF6 be?
Xenon hexafluoride
Find the missing mass.
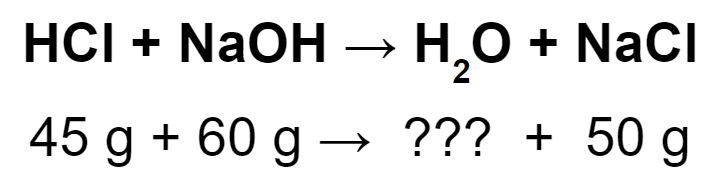
55 g
If more solute can be added to a solution and still be dissolved, what level of saturation would the solution be?
Unsaturated
List each of the following chemicals as an acid or a base.
HBr
NaOH
H3PO4
HBr - Acid
NaOH - Base
H3PO4 - Acid
List 3 countries in Europe
France, UK, Spain, Germany, Ukraine, Austria, Sweden, Norway, etc.
What is the electron configuration of Se (Atomic number 34)
1s22s22p63s23p64s23d104p4
OR
[Ar]4s23d104p4
What would the formula be for ammonium phosphate be?
(NH4)3PO4
Write down the numbers to balance out this chemical equation.
___ B + ___S2 --> ___B2S3
4 B + 3 S2 --> 2 B2S3
At 40 °C, 150 g of KNO3 is dissolved in 100 g of water. What level of saturation is the solution?
Supersaturated
An unknown solution has a higher concentration of [OH-] than [H+]. Additionally, when HCl is added to this unknown solution, it becomes neutralized. Which of these chemicals could this solution be?
a) BaCl
b) KF
c) HNO3
d) KOH
d) KOH (It's a base)
What are the four planets in the Milky Way galaxy that have rings?
Jupiter, Saturn, Uranus, Neptune
Order the following elements with the highest ionization energy to the lowest ionization energy.
Cd, Se, Rh, Fr
Se, Cd, Rh, Fr
What would the scientific name be for C2O7?
Dicarbon heptaoxide
Write down the numbers to balance out this chemical equation.
___ C9H20 + ___O2 --> ___CO2 + ___H2O
C9H20 + 14 O2 --> 9 CO2 + 10 H2O

How many grams of K2Cr2O7 can be dissolved in 100 g of water at 20 °C?
About 10 g
Using the image below, what would the name of the following acids be?
H(BrO3) & HF
1. Bromic Acid
2. Hydrofluoric Acid
What are the first 10 amendments to the U.S. Constitution called?
Answer: The Bill of Rights