Calcium-41
What is a name for H2O?
Water
Dihydrogen monoxide
What are the 3 of the 5 types of reactions?
Synthesis
Decomposition
Single Replacement
Double Replacement
Combustion
What are 2 out of the 3 different levels of saturation in a solution?
Unsaturated
Saturated
Supersaturated
What pH is considered neutral?
7
List all 5 vowels in the English alphabet.
A, E, I, O, U
How many protons and neutrons does phosphorus-32 have?
Protons = 15
Neutrons = 17
What would the formula for Rhodium (II) Chloride be?
RhCl2
Which type of reaction will have 1 reactant?
Decomposition
If a chemical contains the element sodium, will it be soluble or insoluble?
Soluble (Alkali metal/Capital N)
Is the pH of 6.8 acidic, basic, or neutral?
Acidic
How many inches are in 1 yard?
36 inches
In, S, Zr
Zr
What would the name of BrF3 be?
Bromine trifluoride
Find the missing mass.
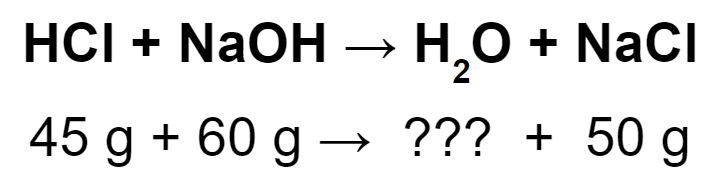
55 g
Is Cs3PO4 soluble or insoluble?
What is the main difference between strong and weak acids?
Strong acids fully dissociate/break apart.
Weak acids do not fully dissociate/break apart.
List 3 countries in Asia
China, Japan, India, Korea(s), etc.
What is the electron configuration of As (Atomic number 33)
1s22s22p63s23p64s23d104p3
OR
[Ar]4s23d104p3
What would the formula be for ammonium carbonate be?
(NH4)2CO3
Write down the numbers to balance out this chemical equation.
___ Al + ___O2 --> ___Al2O3
4 Al + 3 O2 --> 2 Al2O3
At 100 °C, what is the maximum amount of KCl that can be dissolved in 100 grams of water?
60 g KCl
If the [H+] = 1.77 x 10-6 M, what would the pH be?
5.75
-log(1.77 x 10-6 M)
From the water cycle, what is the process of water turning into water vapor called?
Evaporation
Which scientist discovered the nucleus of the atom?
Rutherford
What would the scientific name be for Cl2O7?
Dichlorine heptaoxide
Write down the numbers to balance out this chemical equation.
___ C11H24 + ___O2 --> ___CO2 + ___H2O
C11H24 + 17 O2 --> 11 CO2 + 12 H2O
 At 80 °C, would 20 g of KClO3 in 100 g of water be considered saturated, unsaturated, or supersaturated?
At 80 °C, would 20 g of KClO3 in 100 g of water be considered saturated, unsaturated, or supersaturated?
Unsaturated
Using the image below, what would the name of the following acids be?
H(BrO3) & HF
1. Bromic Acid
2. Hydrofluoric Acid
How many adjectives are in the following sentence?
The green car zoomed quickly through the old road.
2 adjectives
Green (car) & old (road)