Out of: pounds, inches, ounces, and milliliters, this is the only unit that belongs to the metric system
What is millilliters?
What is a liquid?
This type of pure substance, such as Water (H2O), is made of two or more elements chemically combined.
What is a compound?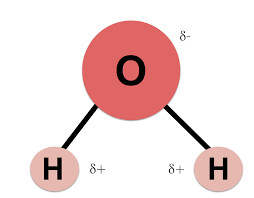
These two subatomic particles are located inside the nucleus of the atom.
What are Protons and Neutrons?
This is the number of valence electrons found in an atom of Nitrogen (Group 15).
What is 5?
Chemical equations must be balanced in order to satisfy this scientific law.
What is the Law of Conservation of Mass?
This force is responsible for holding protons and neutrons together in the nucleus against the repulsion of positive charges.
What is the Nuclear Strong Force?
The formula for density
What is D = M/V or density equals mass divided by volume
A solid melts into a liquid because of an increase in this.
A salad or trail mix is an example of this type of mixture because you can see the different parts.
What is a Heterogeneous Mixture?
These are atoms of the same element that have different numbers of neutrons (and therefore different mass numbers).
What are Isotopes?
This type of bond is formed between a metal and a nonmetal and involves the transfer of electrons (gain and loss)
What is an ionic bond?
On an energy diagram, if the energy of the products is lower than the energy of the reactants, the reaction is classified as this.
What is Exothermic?
The most penetrative form of radiation which can be stopped only by lead and concrete.
What is Gamma?
In an experiment testing how temperature affects reaction speed, temperature is this type of variable because it is changed by the scientist.
What is the Independent Variable?
According to Bernoulli, this decreases as speed increases in a fluid.
What is pressure?
----- DAILY DOUBLE -----
Select how much you would like to wager before answering.
Milk and fog are examples of this specific type of mixture, where particles are larger than those in a solution but do not settle out like they do in a suspension.
This group of elements, found in Group 18 of the periodic table, is known for being unreactive.
What are the Noble Gases?
This is the correct chemical name for the covalent compound S3O2
What is trisulfur dioxide?
A substance that speeds up a chemical reaction by lowering the activation energy required.
What is a Catalyst?
This nuclear process involves splitting a heavy nucleus into lighter nuclei and is currently used in nuclear power plants.
What is Fission?
The equivalent of 200.0 mg in grams (g).
What is 0.2 mg?
On a heating curve, this happens to the temperature while a phase change is actually occurring.
What is remaining constant or not changing.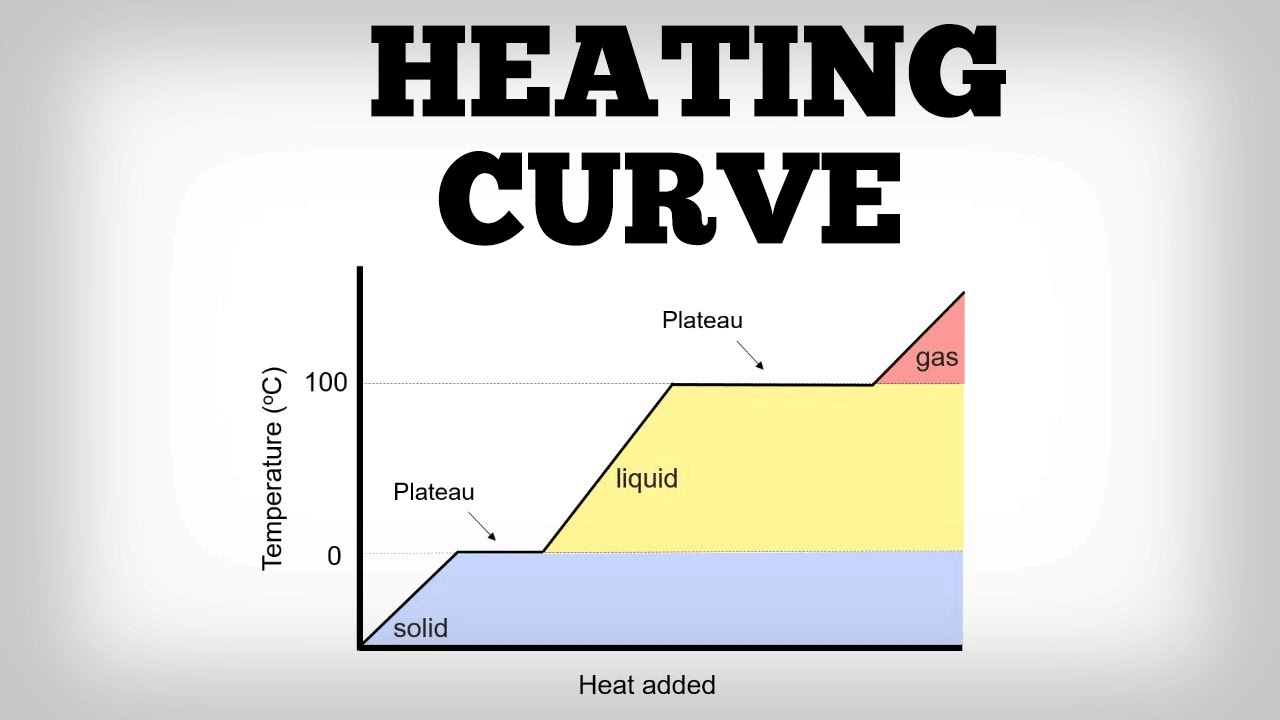
The term that describes the classification of shapes below:

What is a mixture?
This is the number of neutrons in an atom of the isotope of Chlorine-39
(atomic number 17; 39 - 17 = 22)
This is the correct chemical formula for the ionic compound Sodium Phosphide
What is Na3P?
(Sodium forms a +1 charge, Phosphorus forms a -3)
These are the correct coefficients to balance the equation:
___ Na + ___ Cl2 --> ___ NaCl
What is 2, 1, 2 (2Na + Cl2 --> 2NaCl)
If a radioactive sample has a half-life of 5 months, this amount of a 40g sample will remain after 15 months.
What is 5 grams? (40g--> 20g --> 10g --> 5g)