H
Ionic bonds take place between what type of elements?
Metal and a non-metal
What part of the atoms contains almost all of the mass?
The nucleus
List the three types of radiation.
Alpha, Beta, Gamma.
Who discovered the electron?
Thomson
Hg
Mercury
Covalent bonds take place between what types of elements?
Two non-metals
What part of the atom do electrons move in orbits around the nucleus?
The shell
The weakest type of radiation.
Alpha Radiation.
Who discovered the nucleus?
Rutherford
Au
Gold
The type of bond where atoms share a pair of electrons.
Covalent Bond
What subatomic particle differentiates one atom from another?
The number of protons.
The strongest type of radiation.
Gamma radiation
Who created this model?
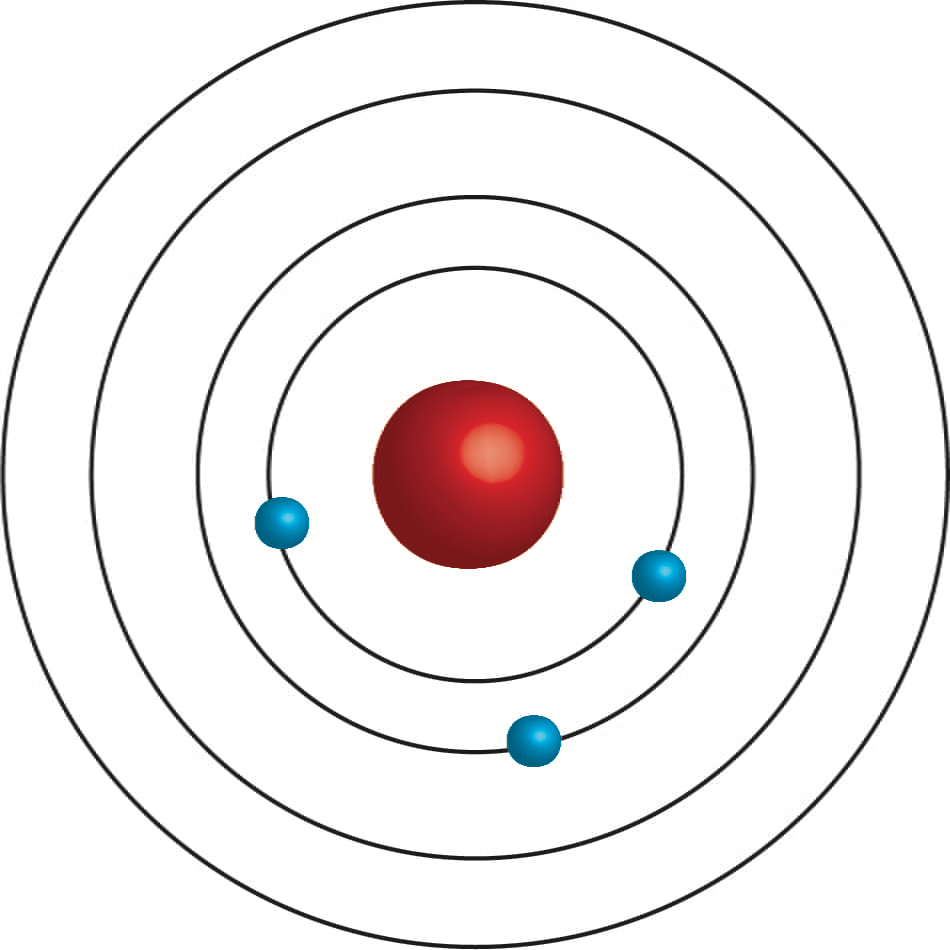
Bohr
Fe
Iron
The type of bond where atoms transfer electrons.
Ionic bond
What term refers to the number of protons in the nucleus?
The atomic number.
Isotopes are atoms of the same element with a different number of _______.
Neutrons
Who proposed the Plum Pudding model?
Thomson
Ag
Silver
What is the exception to the rule: covalent substances form molecules.
Covalent crystals.
What term refers to the number of particles in the nucleus? (Protons + neutrons)
Mass number.
List two applications of radioactive isotopes.
Producing energy, medicine, agriculture, archeology, industry.
Who discovered that electrons orbit at certain distances from the nucleus?
Bohr