The ability to do work.
What is energy?
He created the Periodic table.
Who is Mendeleev?
An electrostatic attraction forms between atoms when they share or transfer valence electrons.
What is a chemical bond?
A molecule made of two atoms (whether of the same element or not).
What is a chemical reaction?
This is the push or pull on an object.
What is force?
Daily Double
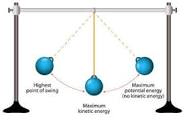
What is kinetic energy?
There are 118 of them.
How many elements have been discovered?
This principle states that atoms generally are most stable when they have eight electrons in their valence energy level.
What is the octet rule?
A combination of chemical formulas and symbols that models a chemical reaction.
What is a chemical equation?
The branch of physics that studies forces and how they can change an object’s motion.
What is dynamics?
Stored energy that can be used later.
What is potential energy?
The scientists who study elements and other materials.
What are alchemists?
A chemical bond formed as a result of two atoms sharing valence electrons.
What is a covalent bond?
SI fundamental unit for the quantity of matter in a substance; a count equal to 6.022 × 1023.
What is a mole?

What is contact force?
This can be used to heat water.
What is solar energy?
These elements are located on the far right side of the periodic table.
What are nonmetals?
This type of molecule is made of two atoms (whether of the same element or not).
What is a diatomic molecule?

Daily Double
What is an example of a chemical change?
The tendency of matter to resist changes in its motion.
What is inertia?
The sum of any kinetic energy and all the forms of potential energy in a system.
What is mechanical energy?
An electron found in the outermost energy level of a neutral atom.
What is a valence electron?
Bonds that form between a metal and a nonmetal.
What is an ionic bond?
This is the mass of one mole of a substance.
What is molar mass?
A force that acts between objects that are not touching; also called force at a distance.
What is a field force?