I am one type of atom.
What is an element?
When water molecules are attracted to one another.
What is cohesion?
*Acids have a pH ___ than 7.
Less than 7
I am what appears on the left of the “yield” sign in a chemical equation
What is a Reactant?
*How many atoms of each element are in Methane?
CH₄
C-1
H-4
I am a chemical substance made of two or more elements bonded togther.
What is a compound?
When water is attracted to other substances
What is adhesion?
* Bases have a pH ___ than 7.
greater than 7
I am what appears on the right of the “yield” sign in a chemical equation
What are products?
*How many atoms of Oxygen are in this compound?
2H₂SO₄
8 atoms of Oxygen
Cereal is an example of this type of mixture.
What is a heterogeneous mixture?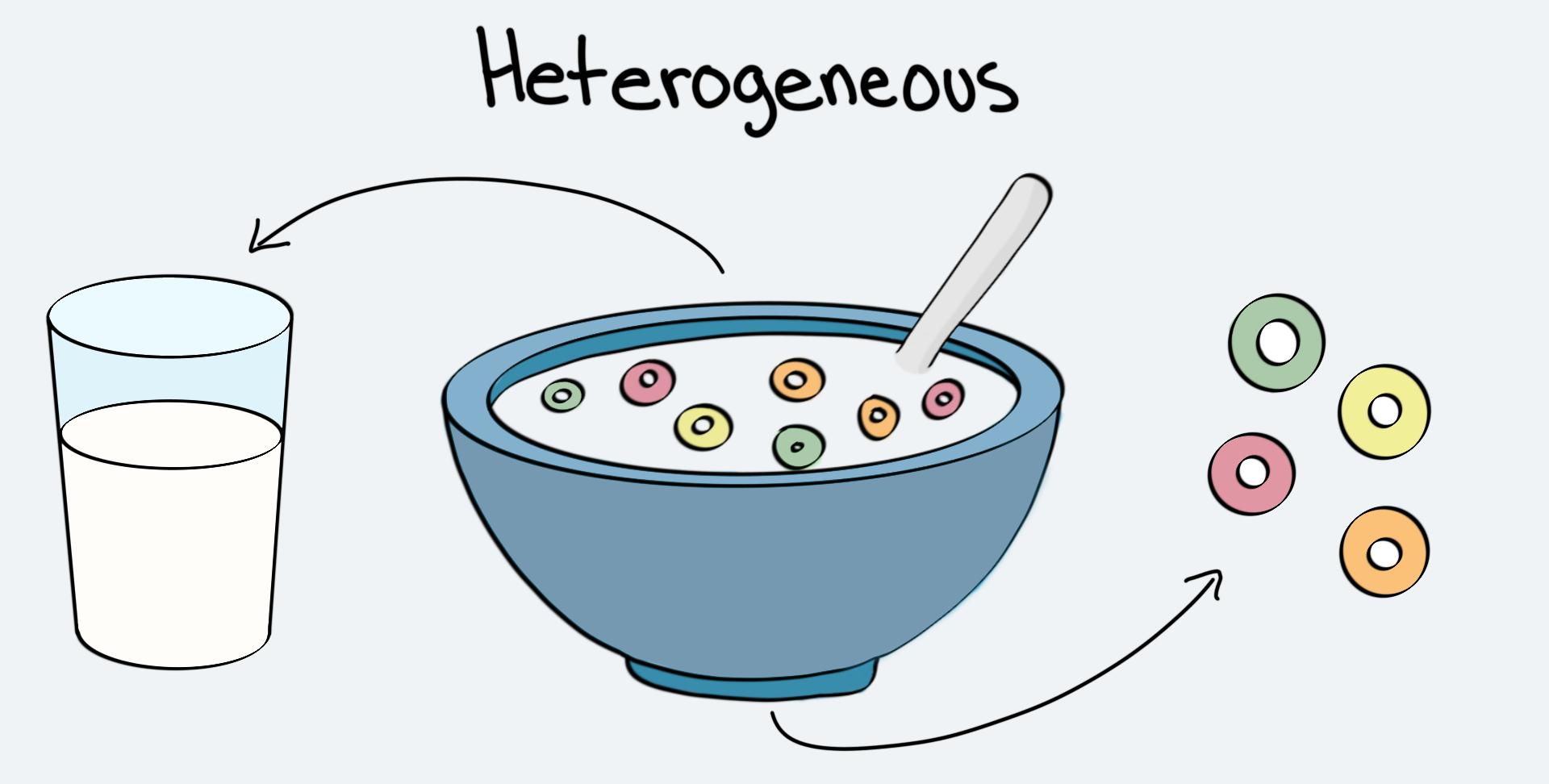
The property allows the Water Strider to walk on water
What is surface tension?
Lemon juice and vinegar are examples of this.
What are acids?
Atoms aren't created or destroyed in a chemical reaction they are ____.
What is rearranged?
*How many atoms of Oxygen are in the reactants side and products side?
Reactants:10 atoms of Oxygen
Products:10 atoms of Oxygen
Sweet Tea is an example of this type of mixture.
What is a homogeneous mixture?
These properties are used when water droplets form on a leaf
What are cohesion and adhesion?

Soap and baking soda are examples of this.
What are Bases?
*If 12G of Hydrogen reacts with 96G of Oxygen, what is the total mass of water produced?
108G of water
*How many of each element are in this equation?
PCl5 + H2 O ⟶ POCl3 + HCl
P: 1 P: 1
Cl: 5 Cl: 4
H: 2. H: 1
O:1 O: 1
Classify Carbon Dioxide, Orange Juice with PULP, Oxygen, and Saltwater.
What is an example of a compound, heterogeneous mixture, element, and homogeneous mixture?
*Explain how adhesion and cohesion help water travel from roots to leaves in plants.
Cohesion keeps water molecules together, adhesion helps them stick to the plant's walls and move upward.
*Compare acids and bases in terms of taste and reactivity.
Acid tastes sour and can corrode metals.
Bases taste bitter and feel slippery.
*Is this equation balanced or unbalanced?
Unbalanced
*How many each element is in this equation? Balanced or unbalanced?
Fe + H2 O ⟶ 3Fe3 O4 + H2
Fe:1 Fe:9
H:2 H:2
O:1 O:12
Unbalanced