All atoms are made of 3 subatomic particles called?
Protons, Neutrons, and Electrons
Its name means indivisible. It is the building block of matter
an atom
Which of the following is a compound?
Na
H2O
N
Cl
H2O
Combustion is a common type of what kind of change?
Chemical
What chemical reaction has taken place in this picture?

Rust
This state of matter has particles in constant random motion.
Gas
An atom is described as a nucleus of protons and neutrons surrounded by what?
Electron Cloud
Give me an example of an element?
Anything from the periodic table
Define what a pure substance is
an element or compound made up of only one type of atom
What is this scientific idea called?
The mass of the reactants is equal to the mass of the products
Law of Conservation of Mass
Which of these is not an example of a chemical change?
burning wood
souring milk
baking a cake
boiling water
boiling water
In this state the particles have strong molecular attraction
Solid
Electrons orbit around the nucleus and have a what kind of charged?
Negative
Which of these is an example of a mixture
Water (H2O)
Sugar
CO2
Popcorn and salt
Popcorn and salt
Is water, H2O, a mixture or a compound? Why?
compound, because it is chemically combined
How can you be sure that the Law of Conservation of Mass is preserved in reaction
Use a closed container
Which of these could be classified as a chemical property of matter?
odor
melting point
molecule size
generation of heat
generation of heat
Which order of the diagrams indicates DECREASING distance between atoms?
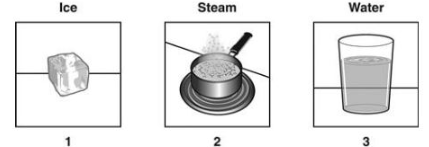
2, 3, 1
All protons have what kind of charge and have a mass of about 1amu
Positive
Draw a picture of mixture
Varies, two or more elements combined physically
Elements are pure substances that make up all matter. Gold, aluminum, iron, and lead are some examples of these pure substances. What is the basic building block of all pure substances that make up matter in the world?
Atoms
CuCl2 + Al ---> AlCl3 + Cu
Balance the single replacement chemical reaction.
3CuCl2+2A ---> 2AlCl3+ 3Cu
Which of these elements is nonmetallic and a gas at room temperature?
silicon
sodium
nitrogen
potassium
Nitrogen
Solids are often defined as having a definite shape, while gases and liquids have no definite shape. This can best be explained by the fact that?
solids have a more ordered arrangement of atoms and a stronger molecular attractiom
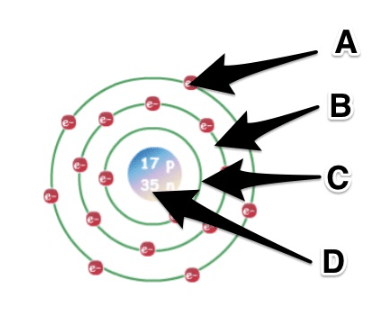
What letter represents the mass of the oven?
D
What is the difference between atoms and molecules?
Atoms are an element's most basic unit whereas molecules are chemically bonded together.
A salad including lettuce, tomatoes, and onions would be classified as a
Mixture
A balanced chemical reaction obeys the law of what?
Conservation of Matter
This column of the periodic table represents the halogen family. This is a family of reactive elements. Compare and contrast how the elements are similar and different.

different atomic number
different mass number
same number of valence electron
What happens to the atoms of matter as they change state?
As matter moves from a solid to a gas, atoms gain energy and move apart