How many elements are there in a compound?
2 or more
Who Am I?
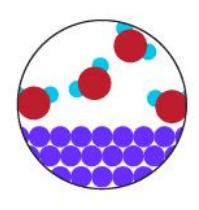
What is a Heterogenous Mixture?
This is what happens when water sticks to a spiderweb after a rainstorm.
What is Adhesion?
In a chemical reaction, what happens to the number of atoms?
A. Atoms are lost during the reaction.
B. The number of atoms remains the same.
C. Atoms multiply during the reaction.
D. Atoms are only rearranged but not conserved.
B
What is on the right side of the periodic table?
Metaloids
I am a molecule of an ____________ of oxygen, What am I?

What is a Element?
What Am I?
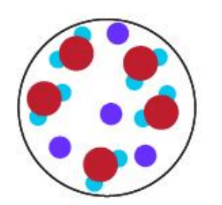
What is a Homogenous Mixture?
This is what happens when water droplets attract one another and form a larger pool of water.
What is Cohesion?
In the reaction 3Fe + 4H2O → Fe3O4 + 4H2, which statement best describes
what happens to the atoms?
A. Iron atoms are created.
B. Oxygen atoms are destroyed.
C. Atoms are rearranged into new combinations.
D. The total number of atoms decreases.
C
How many Element are in the equation?
Fe + CuSO₄ → FeSO₄ + Cu
4
I am a __________ of Carbon Dioxide?
What am I?
What is a Compund?
What type of mixtures are provided in the pictures below:

What is a Heterogenous Mixture?
What is the action called when water sticks to water which sticks to the flower and moves in an upward motion?
What is Capillary Action
The decomposition of hydrogen peroxide is represented by the equation below:
H2O2 → H2O + O2
Which of the following best describes this equation in terms of atom conservation?
A. The equation is correct because there are equal atoms of each element on both sides.
B. The equation is not correct because there are fewer hydrogen atoms on the left than on the right.
C. The equation is correct because the number of molecules on both sides is the same.
D. The equation is not correct because there are fewer oxygen atoms on the left than on the right.
D
How many Elements are in the equation?
2Ca₃(PO₄)₂ + 6SiO₂ → P₄O₁₀ + 6CaSiO₃
4
Who am I:
What is a Compound?
I am a Homogenous Mixture and I am all around you, and I have enough volume to fill a ballon, and your lungs too! What AM I?
What is Air?
Rain water falling into droplets
What is Cohesion?
In industrial processes, the reaction Na3PO4 + 3HCl → 3NaCl + H3PO4 is crucial. Select TWO correct statements.
A. The number of sodium atoms remains constant.
B. The number of phosphorus atoms increases.
C. The total number of atoms stays the same.
D. The number of chlorine atoms increases.
E. The number of hydrogen atoms decreases.
A C
What are the elements in the equation?
Fe + CuSO₄ → FeSO₄ + Cu
Iron, Copper, Sulfur, Oxygen
Who am I?
What is a Element?
I am a mixture that has a uniform appearnance and does not show seperate parts.
What is a Homogenous Mixture?
When water droplets form due to cohesion I am the thin film that forms at the top. What am I?
What is Surface tension
In the reaction:
CH4 + 2O2→CO2+2H2O
If 16 grams of methane (CH4) reacts with 64 grams of oxygen (O2), what is the total mass of the products?
80
What are the elements in the equation?
2Ca₃(PO₄)₂ + 6SiO₂ → P₄O₁₀ + 6CaSiO₃
Calcuim, Phosphorus, Oxygen, Silicon