Surprise $500 points!
What are the 3 subatomic particles?
Protons, Neutrons, and Electrons
An element is a substance made up of _______ type/s of atom.
A. One
B. Two
C. Three
D. Four
A: One
How many electrons fit into the first four energy levels?
2, 8, 8, 2
Electrons in the outer most energy level are called __________.
Valence Electrons
What is a foley?
An audio recording using random objects as sound effects.
What are the charges of the 3 subatomic particles?
Protons - Positive
Electrons - Negative
Neutrons - No charge
What is the name of the two numbers listed here?
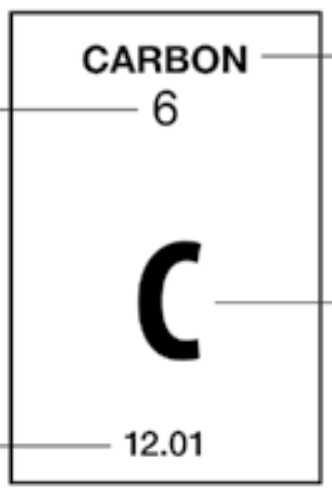
Atomic Number and Atomic Weight (or Atomic Mass)
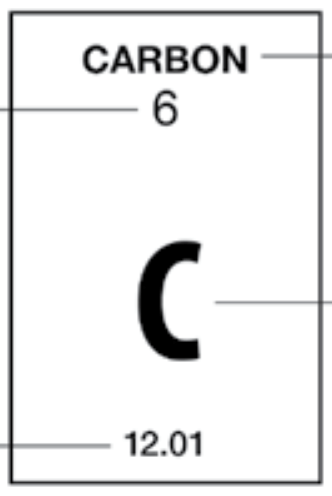
Which element is this?

Oxygen
2x points!
In a covalent bond, electrons are __________ between atoms.
Shared
What is this image from?
PhET
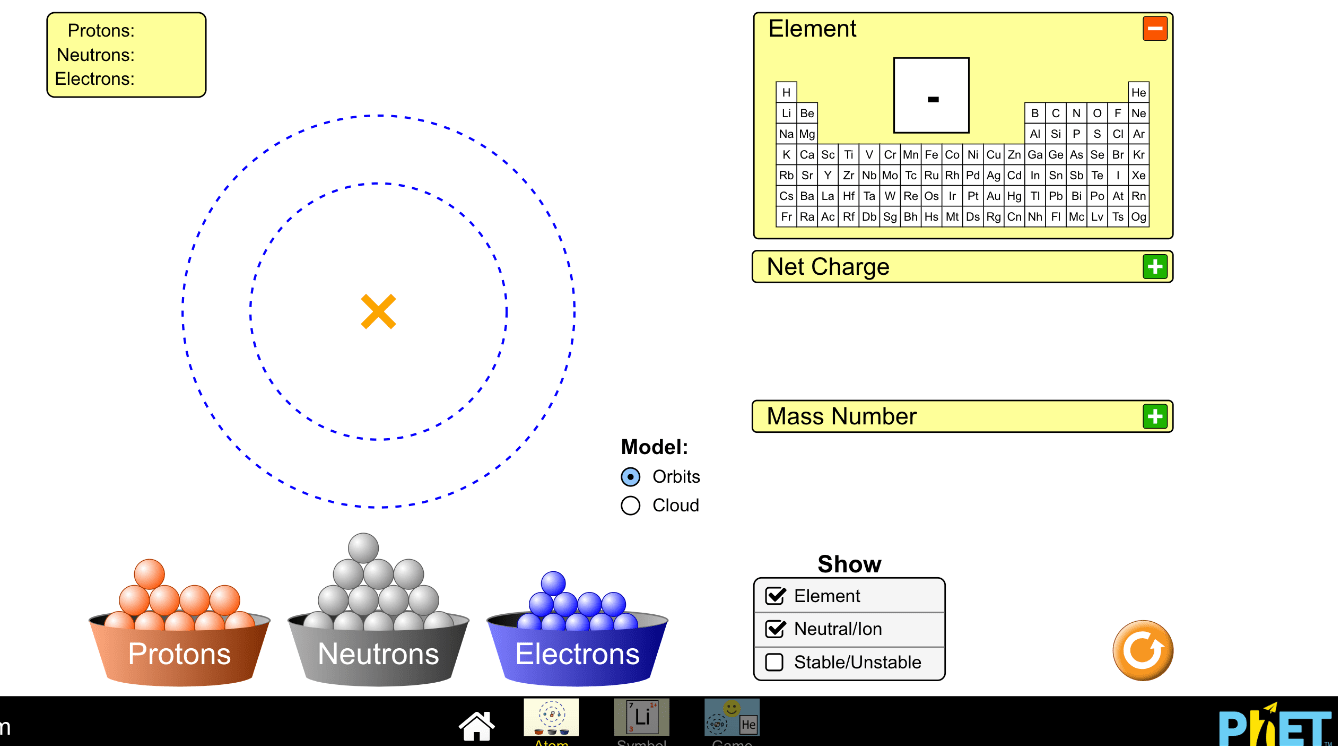
This image shows a model of what? Be specific!
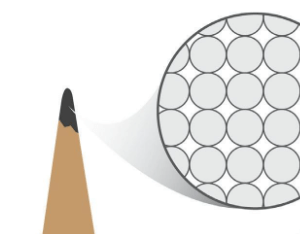
Carbon atoms of a pencil.
What are these called?
Isotopes
Explain how Argon electrons fill their energy levels.
2, 8, 8
Explain what will happen and why it will happen:
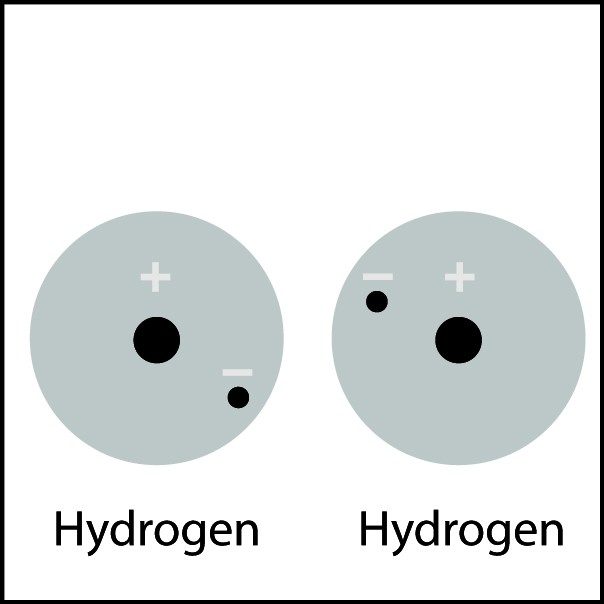
Electrons will be attracted to the protons and the electrons will be shared in order to fill their first energy levels (stability).
Name these YouTube stars

Paige and Dexter
True or False: These two objects would be attracted to each other.
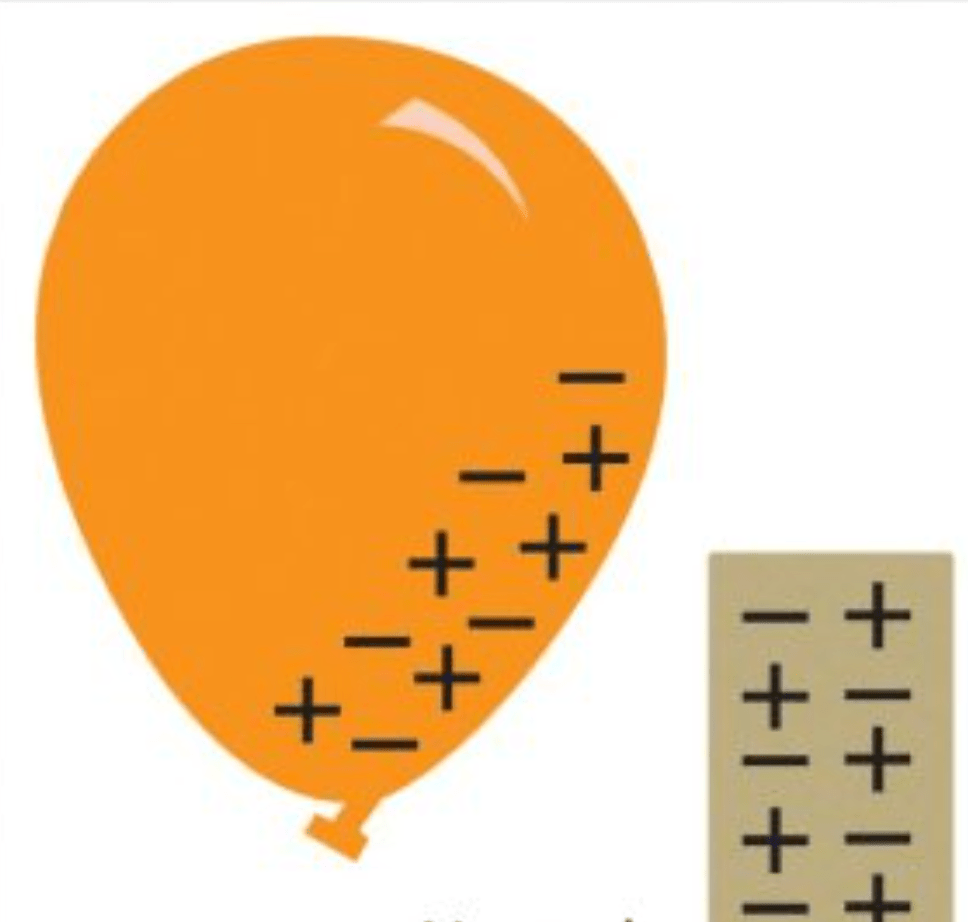
False
How many neutrons does Boron have?
6
True or False: Helium is a stable atom based on the electrons filling its energy level.
True
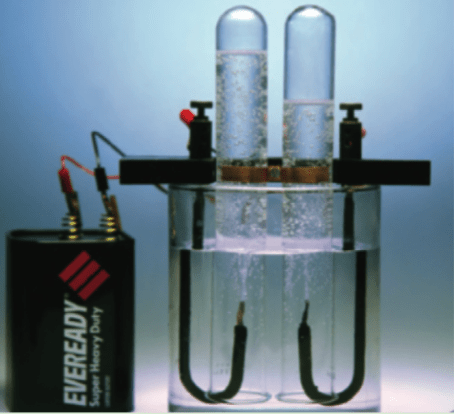
What is happening in an Electrolysis Experiment?
How many current subscribers does Mr. Carlson have?
76! Give me my award!!

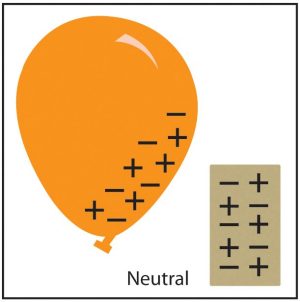
The balloon has been rubbed against a shirt and electrons have accumulated. What will happen when brought near the water?

Electrons built up on the balloon creating a negative charge. The electrons pushed the electrons from the water away (same charges repel). This left a positive charge in the water and, thus, an attraction between the balloon and the water.

True or False: Oxygen has 8 electrons in its nucleus.
False. Electrons are not in the nucleus.
Hydrogen, Lithium, and Magnesium share what characteristic about their electron energy levels?
Their outer energy level is NOT FILLED.
Do these atoms form a covalent bond or an ionic bond? Explain WHY in order to get this correct.
Ionic Bond because sodium (Na) wants to lose its electron in order to be stable, and chlorine (Cl) wants to gain one in order to be stable.
What does PhET stand for?
Physics Education Technology