What is a compound?
two or more different types of atoms
What is Charles' Law?
When gas temperature increases, its volume increases.
The movement of an object from one place to another.
motion
what is radiation?
Transfer by electromagnetic waves
When boiling water on a stove, heat moves from the burner to the pot by _______ and from the pot to the water by _______.
conduction, convection
What is a molecule?
two or more atoms held together by chemical bonds
State of matter with no definite shape or volume
gas
What is the ability to make things move or cause change?
energy
When thermal energy is added to a substance, particles:
Spread farther apart and speed up
Heat movement by fluid motion
convection
is chicken noodle soup homogenous or heterogenous?
heterogenous
What is the effect of thermal energy on particle movement
more thermal energy, faster movement
Energy of movement.
kinetic energy
What slows or stops heat transfer?
An insulator
When you stretch a spring or rubber band, what kind of energy are you giving it?
elastic potential energy
What makes up all matter?
atoms
What happens when a liquid freezes?
Particles slow down and move closer together.
A push or pull that can change an object’s direction or speed.
force
energy always moves:
hot to cold
Since the moon's gravity is less than Earth's, all objects, including mammals, have different weights on the moon than on Earth. Use the table below to calculate how much a dog weighs on the moon.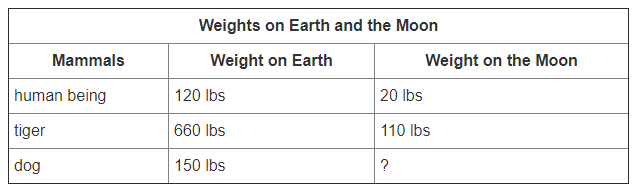
25
Which measurement tells how much matter is in an object?
Mass
What is Boyle's Law?
As pressure increases, gas volume decreases.
Using force to move an object a certain distance.
work
Transfer of energy through direct contact
conduction
Stored energy an object has because of its height.
gravitational energy