This state doesn't move much, it really just vibrates in place.
What is a solid?
Two or more things are mixed together. If one thing dissolves then it must be this.
What is Homogenous?
The density of water. It's pretty easy to remember this one!
What is 1 g/mL or 1 g/cm3?
The carpet feels very soft on my feet.
What is texture?
When describing the movement of particles, you are actually describing this type of energy.
What is kinetic energy?
During this phase particles will move around freely but still touch allowing the matter to maintain its volume.
What is a liquid?
We identify a mixture of grapes, blueberries and strawberries as this.
What is heterogenous?
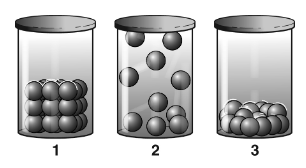
This item is clearly the least dense.
What is the square or cube?
An objects ability to sink or float in a liquid.
What is density?
Solids and Liquids have this in common.
What is definite volume?
Name these states of matter in order.
What is solid, gas, and liquid?
A chocolate kisses are best described as this type of mixture.
What is homogenous?
This is clearly the most dense.
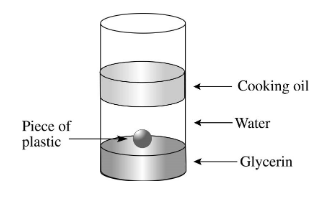
What is Glycerin?
This properties identifies how heavy something is by comparing it to something else, often by using a balance.
What is mass?
This is just a change in state.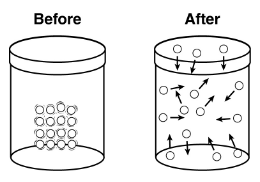
What is solid to gas?
Describing the physical properties of a gas is as simple as size and shape.
What is indefinite volume and indefinite shape?
Don't be tricked by sugar, it's clearly this.
What is a pure substance?
If a liquid has a density of 1.21 g/mL and you pour water into the same container the water will most definitely do this.
What is float or layer on top?
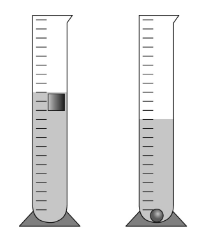
The picture shows one way to measure this physical property.
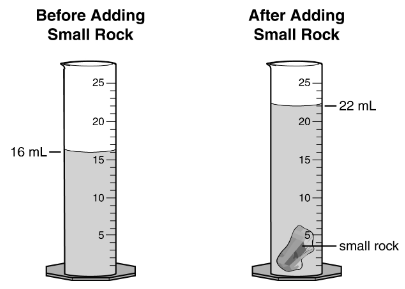
What is density?
A rock is placed in a graduated cylinder with 50 mL of water. The water rises to 56 mL. So therefore the rock has a density of...
What is a density of 6 mL?
When you add heat to matter you are also causing this to happen to it's particles.
What is an increase in kinetic energy or movement?
A heterogenous mixture is best defined by this one word, which the Greeks used hetero- to describe.
What is different?
If an item has a density of 0.97 g/mL it will obviously do this when placed in water.
What is float?
A baseball has a definite shape and volume.
What is a solid or What is describing its State of Matter?
This sample has the least kinetic energy of the three.

What is sample Z (solid)?