Stoich!
Stoich!
Chemical Bonding
I've got the Solution!
Acids & Bases
Gases
Used to determine moles of a substance in the balanced chemical equation.
What is a coefficient?
One of these is not a type of electromagnetic radiation.
radiowaves, microwaves, sound waves, infrared, visible
What are sound waves?
CaCl2 Li3N Al2(SO4)3 all exhibit this type of bonding
What is Ionic bonding?
This is another name for a SOLUTION.
What is "A HOMOGENEOUS MIXTURE?"
HCl HNO3 H2SO4 vinegar
Identifies as...
What is an acid?
This is the relationship between pressure and volume.
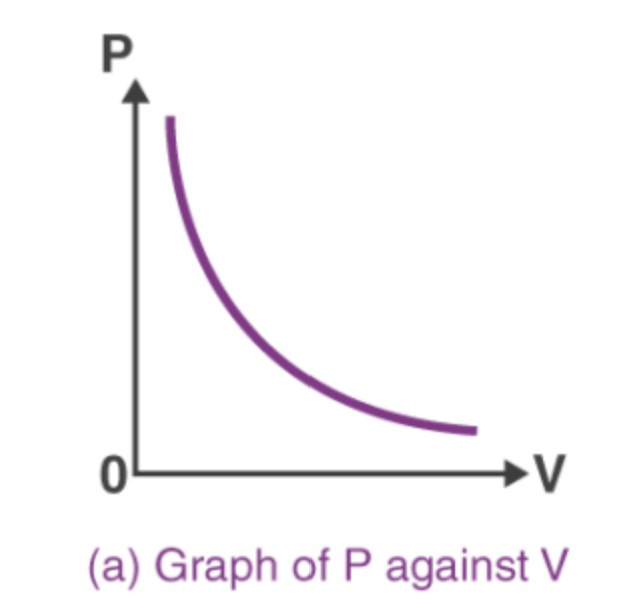
What is INDIRECT (or INVERSE)?
The mole ratio of oxygen to carbon dioxide:
C3H8 + 5 O2 --> 3 CO2 + 4 H2O
What is 5 : 3?
This is the relationship between wavelength and frequency.
What is inverse relationship?
(long wavelength, low frequency)
CO2 NH3 BF3 PCl5 all exhibit this type of bonding
What is a covalent bond?
Lemonade solution -- sugar dissolved in water.
This is the solute.
What is "SUGAR?"
What is barium hydroxide?
A gas occupies 4.5 L of space at standard pressure. The pressure is then reduced to 325 mmHg.
This is an example of his gas law.
What is Boyle's Law?
The balanced chemical equation:
__ H2SO4 + __ Al(OH)3 --> __ Al2(SO4)3 +__H(OH)
What are: 3, 2, 1, 6?
This is what the atomic number represents for an atom.
What is number of protons and number of electrons in a neutral atom?
The type of molecular geometry (shape) that has three bonding domains around the central atom.
What is trigonal planar?
The amount of grams of KNO3 able to be dissolved in 100 g H2O at
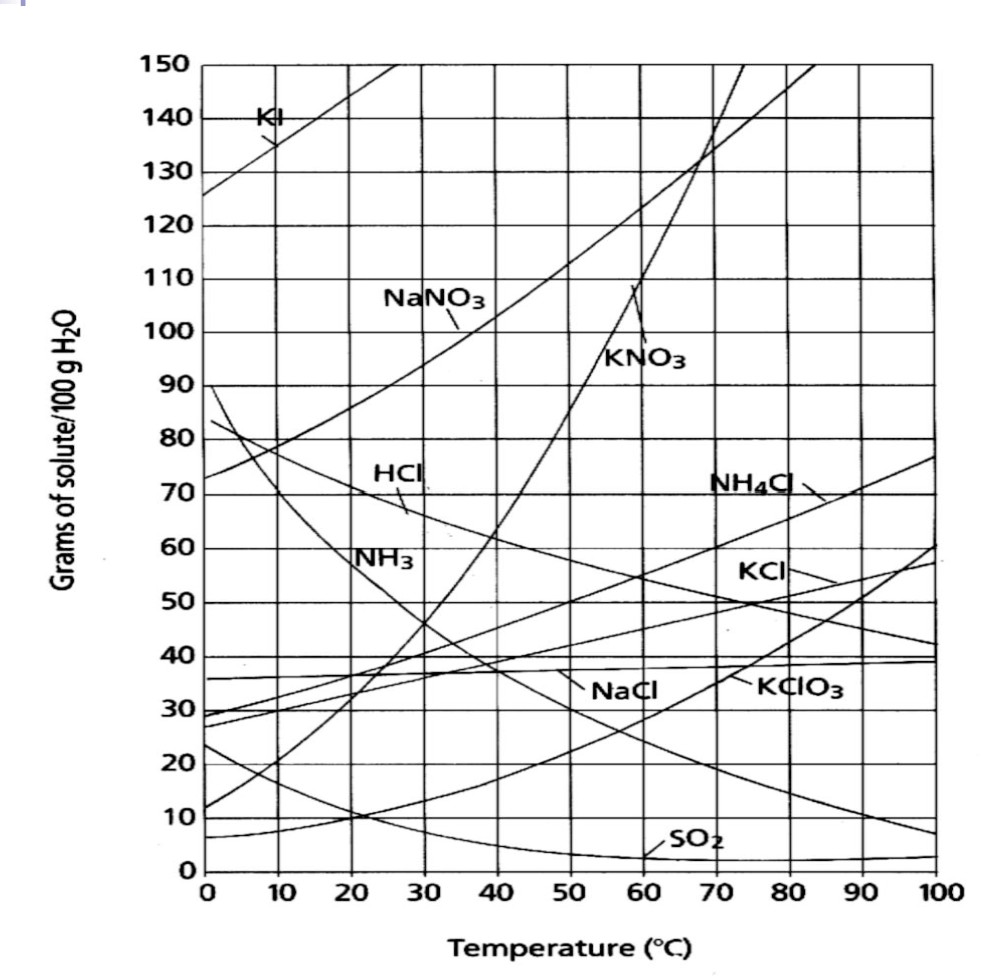
What is 55 g of KNO3 ?
The 2 products from an acid base neutralization reaction.
What are SALT and WATER?
This model is used to describe the behavior of gases, particularly the physical properties @ the molecular level.
What is the Kinetic Molecular Theory?
Known as the reactant that gets all used up and runs outs at the end of the reaction.
What is a limiting reactant?
 Name the element.
Name the element.
Who is PHOSPHORUS?
The first step (once you have the chemical formula) of a Lewis Structure.
What is "determine the number of valence electrons?"
This is the number of moles of potassium iodide, KI, needed to be added to water to produce 2.5 L of a solution with a concentration of 0.35 M.
What is 0.875 moles of KI?
The pH of an acid whose [H+] is 2.67 x 10-3 M.
What is pH 2.57?
A balloon is cooled from 300 K to 100 K. If its original volume was 6 mL, this is its new volume.
What is 2 mL?
A party favor bag is to contain: 3 lollipops, 10 starburst, 1 king size candy bar, 8 tootsie rolls, and 1 ring pop.We have 22 lollipops, 22 starburst, 8 king size candy bars, 32 tootsie rolls, and 5 ring pops. The theoretical yield is.
What is 4 party favor bags?
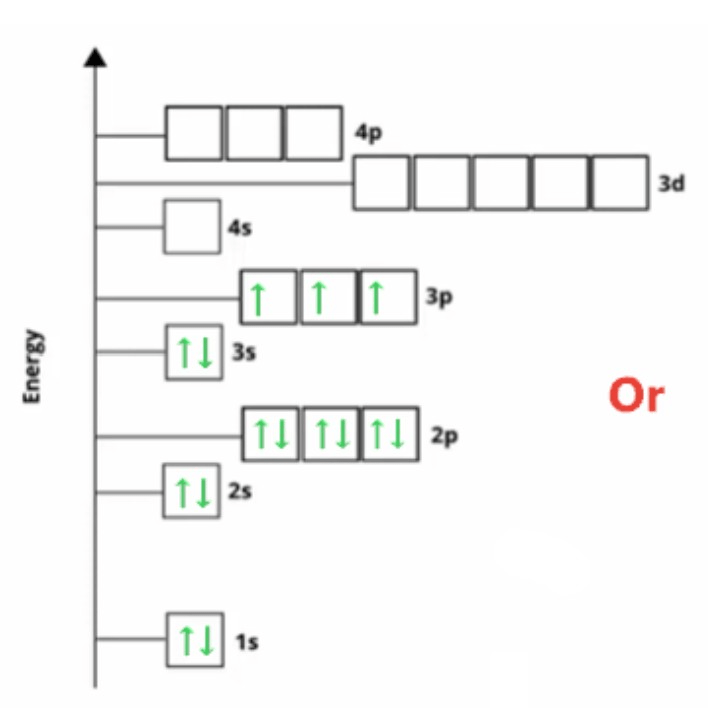
Name the element with the following electron configuration: 1s22s22p63s23p64s23d104p3
Who is As - Arsenic?
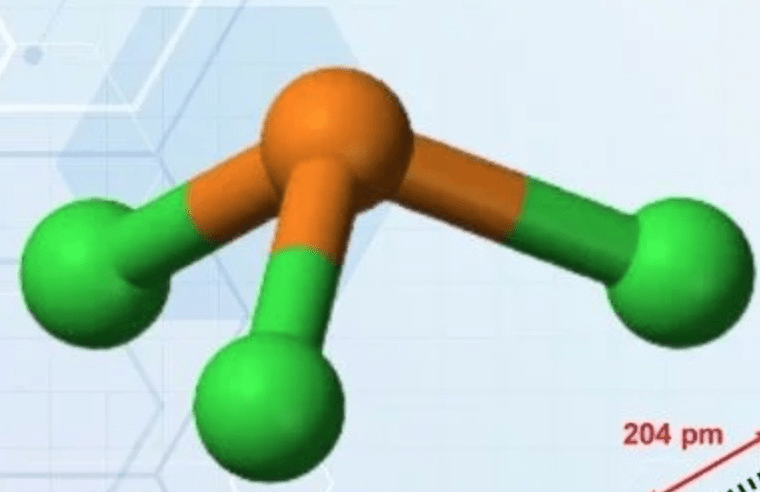
The molecular geometry (shape) for PCl3.
What is "TRIGONAL PYRAMIDAL?"
This is the amount of grams of potassium iodide, KI, needed to make 200 mL of a 2.5 M solution.
What is 83 grams?
The pH of a solution with an [OH-] = 3.43 x 10-8M.
What is pH 6.54?
A sample of helium gas has a volume of 33.6 L at STP. This is the number of moles of the gas.
What is 1.50 moles?
Use PV = nRT
R = 0.0821 L atm / mol K