The measure of the atomic size
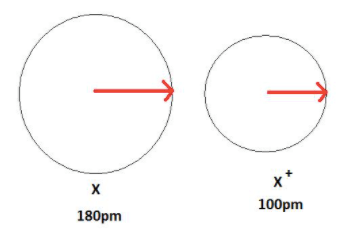
What is atomic radius?
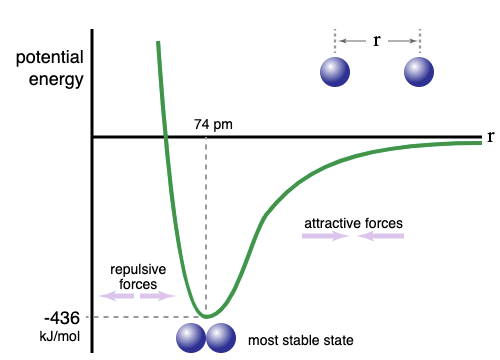
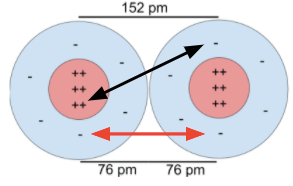
The distance between the nuclei of atoms.
What is the internuclear distance?
The forces between electrons and protons (between opposite charges).
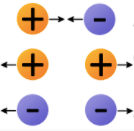
What are attractive forces?
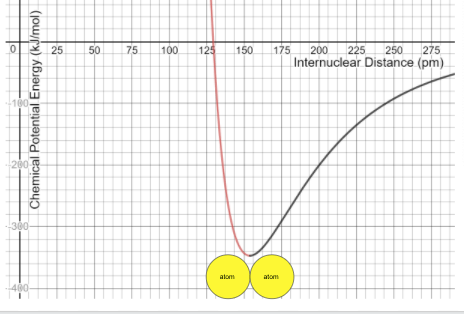
A CPEG diagram shows the relationship between ______ and __________
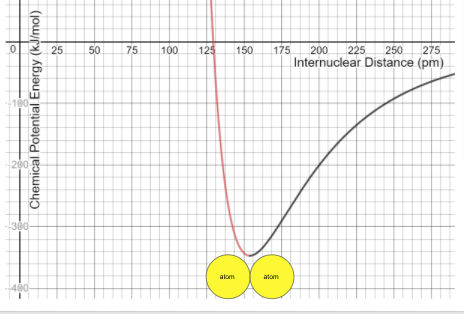
What are internuclear distance and chemical potential energy ?
The unit for atomic radius
What is picometer (pm)?
The internuclear distance where the atoms are stable and the electrostatic forces cancel out.
What is the bond length?
The forces between protons or between electrons (between "like" charges).
What are repulsive forces?
The more negative the energy, the more energy required to _______ the atoms ("break the bond")
What is "separate"?
Approximately the sum of atomic radii.
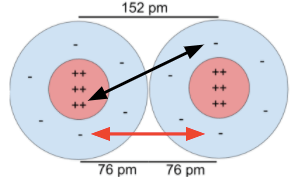
What is bond length?
The potential energy is zero when the atoms are _____ apart
What is far?
The type of forces that dominate when atoms are far apart.
What are attractive forces?
On a CPEG, the variable that determines the strength and stability of a bond.
What is the amount of chemical potential energy in the bond?
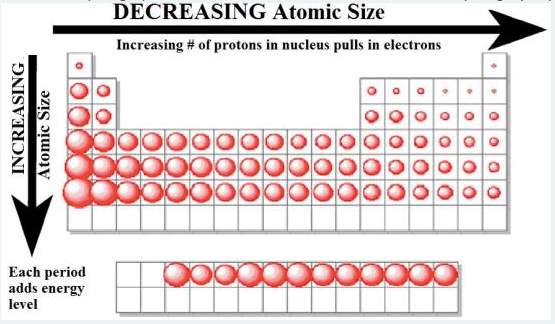
The cause of increasing atomic size down a group/column in the periodic table
What is the number of electrons (or energy levels)?
Atoms in a bond will most likely be found at this energy level.
What is the lowest energy level or energy minimum?
The type of forces that dominate when atoms are closer.
What are repulsive forces?
The (black/ red)_____ atoms require less energy to separate
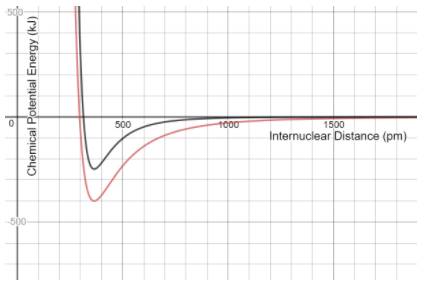
What are black atoms?
The cause of decreasing atomic size across a period/ row in the periodic table.
What is the effective nuclear charge? (or what is the effect of the increasing number of protons?)
The unit used to measure atomic radii, and internuclear distance.
What is picometer (pm)?
At the energy minimum of a CPEG (Chemical Potential Energy Graph), the attractive and repulsive forces between atoms are ______ and ________.
What are equal and opposite?
The (black/red)______ atoms release more energy when forming a molecular bond.
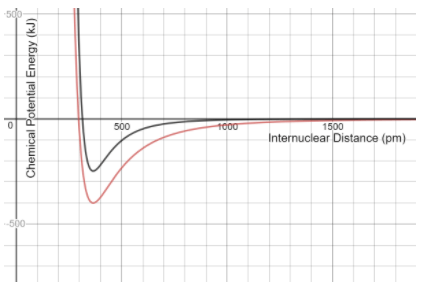
What are red atoms?