What state of matter has the least amount of energy?
Solid
Name all three subatomic particles.
Protons, neutrons, & electrons
True or false? A compound is made up of 2 or more different atoms combined.
True
How many carbon atoms in this formula? C6H1206
Six
Which of these is a physical change: Cutting paper, dissolving sugar in lemonade, melting ice
All of them
What type of chemical reaction absorbs heat?
Endothermic
What does the Law of Conservation of Mass state?
Mass is neither created nor destroyed in a chemical reaction
As temperature increases, atoms will move ________
Faster
The atomic mass number for an element identifies the number of....
Protons & neutrons
Element, compound, or molecule?
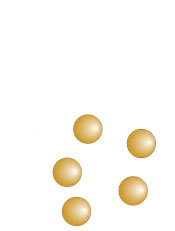
Element
Seven
Which of these is a chemical change: Water boiling, rusting iron, breaking glass
Rusting iron
What type of chemical reaction releases heat?
Exothermic
In a balanced equation, both sides will be __________
Equal in mass
Is air matter? Why or why not?
Air is matter because it has mass and takes up space.
The atomic number of an element identifies the number of....
Protons & electrons
Element, molecule, or compound?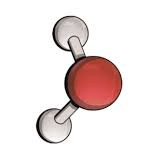
Compound
How many oxygen atoms are in this formula? Al2(SO4)3
Twelve
Name one chemical property of matter.
Examples: Flammability, reactivity, ability to rust, toxicity
2H2+O2→2H2O: This is an example of a chemical _______________
Equation
The left side of a chemical equation is known as the __________
Reactants
Which state of matter won't take the shape of its container?
Solid
What is the center of an atom called?
Nucleus
Element, molecule, or compound?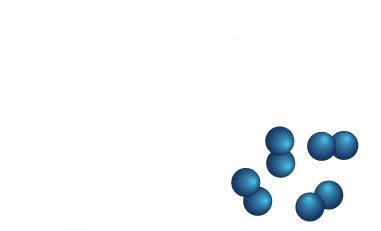
Molecules
How many hydrogen atoms are in this formula? 5NaHCO3
Five
Name one physical property of matter.
Examples: Color, hardness, density, solubility, boiling point, taste, state/phase
The four in NH4 is called a _____________
Subscript
The right side of a chemical equation is known as the _______________
Products
What are the two particles that make up all matter?
Atoms & molecules
If you subtract the atomic number from the atomic mass, you will be able to find...
The number of neutrons
Element, molecule, or compound? CO2
Compound
How many atoms are present in H₂C₂O₄?
Eight
What is the difference between a physical property and a chemical property?
A physical property can be observed without changing the substance, where a chemical property shows potential to create something new.
The 5 in 5CO2 is called a _____________
Coefficient
Balance this equation by finding the missing mass: 5.3g + 8.2g --> ?? + 4.1g
9.4g