All matter is made up of
Atoms
The metric system is based on the number
10
What is an independent variable?
The measured/manipulated variable (the cause)
Name two pieces of laboratory equipment that are used for safety
Goggles, gloves, fire blanket, fire extinguisher
A nail is being removed from a piece of wood. Which type of simple machine is the hammer?
Lever
The positive subatomic particle in an atom is a(n)
Proton
What unit is used to describe volume?
Milliliters (mL)
What is a dependent variable?
The measured variable (the effect)
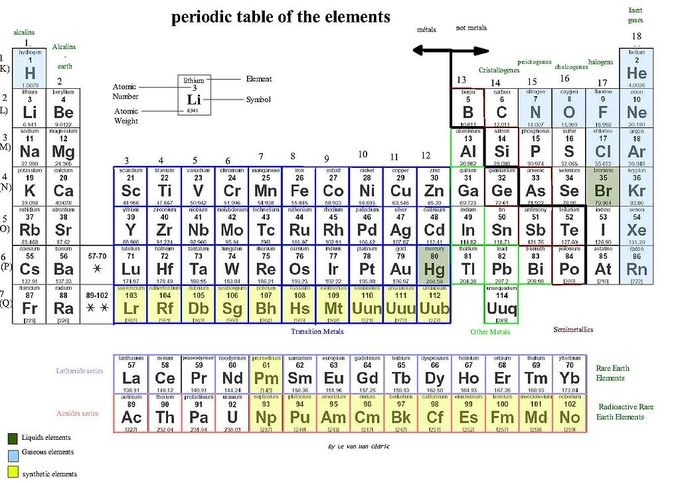
Name an element is considered a metalloid?
B, Si, As, Te, At, Al, Ge, Sb, Po
All of these elements touch the staircase!
Friction is a force that
Causes objects to slow down or stop moving
What happens to water molecules when they are heated?
The move farther apart and move faster
What unit is used to describe length?
Centimeters (cm)
A student is trying to dissolve 20 g of sugar in a beaker containing 250 mL of water at room temperature. What can the student do to make the sugar dissolve faster in the water?
Stir, Shake, or heat the mixture
Name an element that is a Nobel Gas
He, Ne, Ar, Kr, Xe, Rn
A steel ball being rolled down a ramp will push a cup further than a ping pong ball would because a steel ball has
Which subatomic particle is locates outside the nucleus?
Electrons
What unit is used to describe mass?
Grams
Oil floats on top of water, therefore oil must be
Less dense than water
Which element has exactly 19 protons? Use the Periodic Table to find your answer.
Potassium (K)
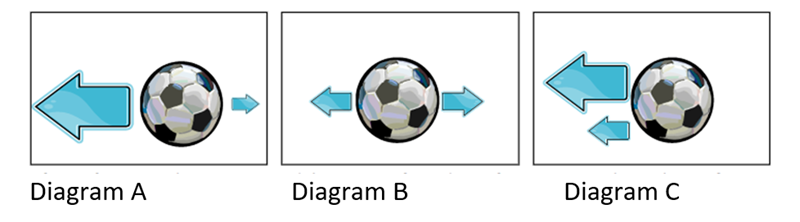
The soccer ball in diagram A will move...
To the Left
Give an example of:
1. A heterogeneous mixture
2. A homogeneous mixture
Answers will vary
Ex:
1. Heterogeneous: trail mix
2. Homogeneous: Lemonade
The density of an object with a mass of 40 g and a volume of 10 cm3 is
Density = Mass/Volume
4.0 g/cm3
The bending of light is know as
Refraction
Which is the best method to identify a solution as an acid or base?
Use Litmus paper or a pH meter to test the pH
What is an example of a chemical reaction?
EX: Adding sodium to acid which bubbles to produce a new substance
*Answers may vary