What is the subatomic particle with a negative charge?
Electron
These particles are not found in the nucleus.
What is the smallest particle of the element silver (Ag) that can still be classified as silver?
Atom
Name the three main states of matter.
Solid, Liquid, Gas
What is the formula for density?
Mass / Volume
Which subatomic particles can be found in the nucleus of an atom?
Protons and neutrons
If an atom has 35 protons in its nucleus, how many electrons will it normally have?
35
A _______ is a group of two or more atoms that is held together by bonds.
Molecule
Substances have more kinetic energy in the _______ state than in the _______ state.
1) gas, liquid
2) liquid, solid
What is the density of a substance with a mass of 10g and a volume of 5 mL? Include units!
2 g/mL
What is the charge of an atom with 4 protons, 4 neutrons and 4 electrons?
Neutral
What atom/element has 35 protons in its nucleus?
Bromine
What is the atomic mass of iron?
![]()
55.845 (exact) or 56 (rounding)
Which of states of matter have a definite shape?
Solid
What is the density of a box measuring 2 inches by 5 inches by 2 inches and a mass of 50 grams?
2.5 grams / mL
Which element has the least number of protons?
Hydrogen
DAILY DOUBLE!!!!!!!
True or False - Anything that has mass and volume is made out of atoms.
TRUE - ALL MATTER IS MADE OUT OF ATOMS!!!!
What element has a greater atomic mass: Plutonium (Pu) or Americium (Am)?
Plutonium (Atomic Mass - 244)
If enough heat is taken away from the container of water, it will _________.
freeze/turn into a solid
To determine the density of an irregularly shaped object, a student dropped the object into 20 mL of H2O. The graduated cylinder rose to 28 mL of H2O. If the object had a mass of 56 grams, what was the density of the object? Include units!
7 g/mL
What is the charge of an atom with 11 protons, 11 neutrons and 10 electrons?
Positive
Draw an atom of helium.

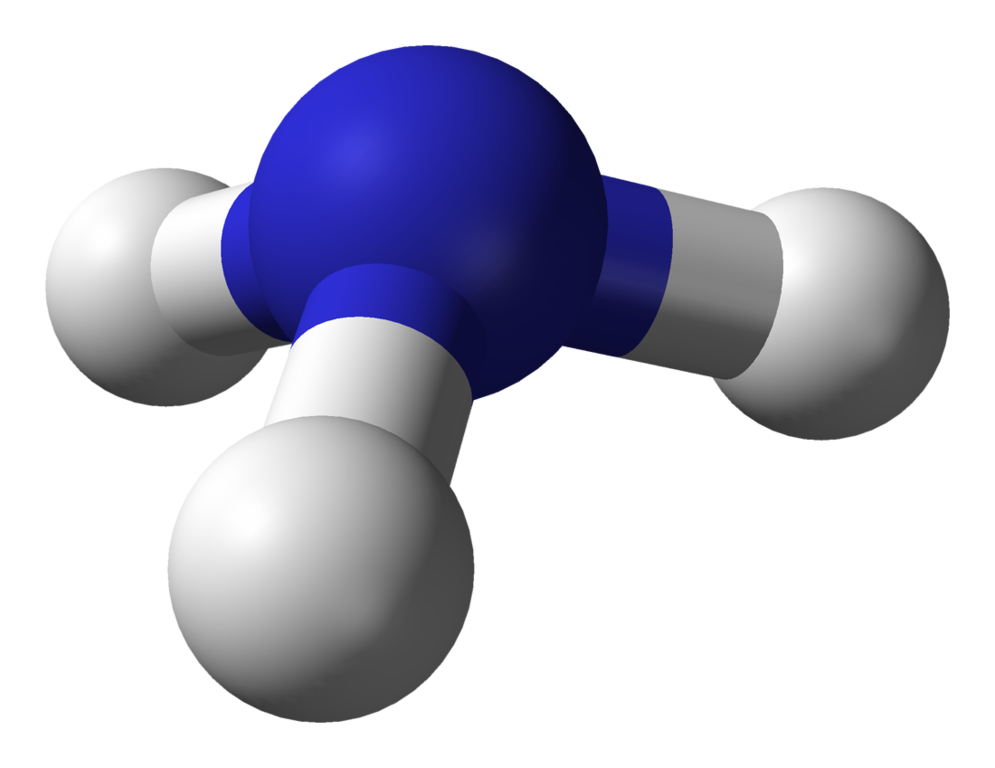
Draw a ball and stick model of NH3.
What phase change is being shown below?
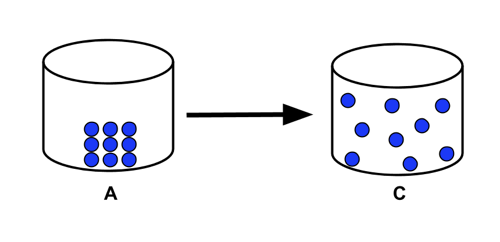
Sublimation (solid to gas)
List each object from highest to lowest density.
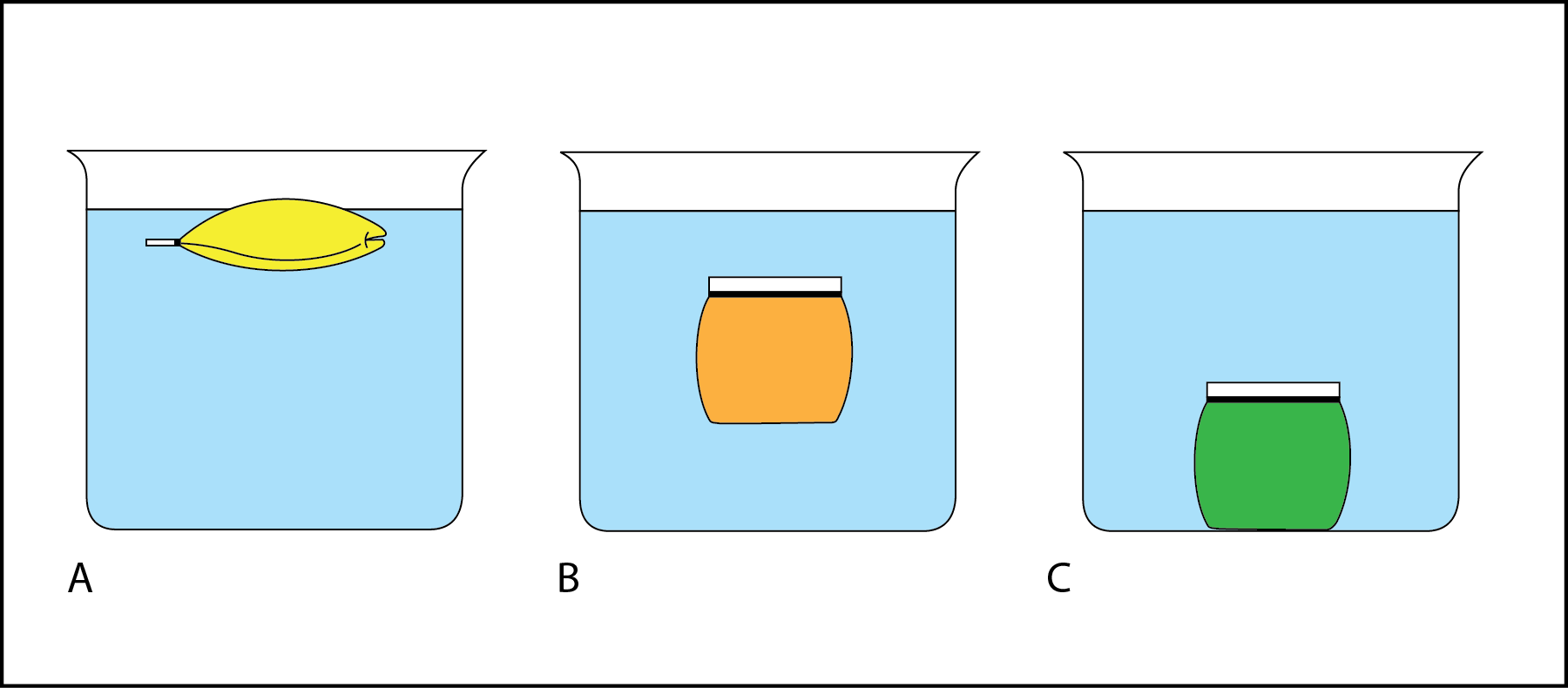
C, B, A