What does a claim do in scientific writing?
It answers the guiding question with a clear, specific statement.
Which group of the periodic table are the Alkali Metals found in?
Group 1.
What group are the halogens in?
Group 17.
Who first suggested that matter was made of indivisible particles called atomos?
Democritus
What element makes up about 75% of normal matter in the universe?
Hydrogen.
Give one example of a type of evidence you can use besides lab data.
Computer simulations, textbooks, class notes, websites, or personal experiences.
Why is sodium stored under oil instead of in water?
It reacts violently with water.
What makes transition metals especially useful in construction?
They are strong, durable, and good conductors of heat and electricity.
What was J.J. Thomson’s atomic model called, and what particle did he discover?
The “Plum Pudding Model”; he discovered the electron
What everyday product contains sodium chloride (NaCl)?
Table salt.
What part of CER connects the data to the claim?
The Reasoning.
Name one real-world use of lithium or potassium.
Lithium: rechargeable batteries.
Potassium: essential for nerve function in the body.
Why are noble gases sometimes called “inert gases”?
inert describes a substance that is non-reactive
They are very stable because their outer electron shells are full, so they rarely react.
Which experiment led Rutherford to propose a dense, positively charged nucleus?
The Gold Foil Experiment.
What metal is liquid at room temperature?
Mercury
Why is it important to explain why your evidence supports your claim?
To show the logic and scientific principles behind your claim, making it convincing and accurate.
Compare the reactivity of Group 1 and Group 2 metals. Which is more reactive?
Group 1 (alkali metals) are more reactive than Group 2 (alkaline earth metals).
What groups are the transition metals in?
Groups 3-12
This atmoic model is from which person?
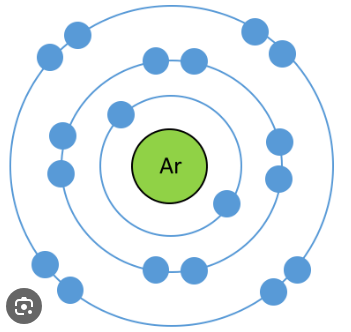
Neils Bohr
What’s the only letter(s) not in the periodic table
J and Q
In the Murder Mystery Game who was the individual who was guilty
Dr. White
Why is hydrogen considered an “oddball” even though it’s in Group 1?
It has 1 valence electron like alkali metals, but it’s a nonmetal with very different behavior.
Elements that have properties of both metals and non metals are called what?
Metalloids
In the quantum model, what do orbitals represent?
Regions of space where electrons are most likely to be found.
In Pokémon, what type is “Arbok” most associated with that links to chemistry?
Poison Type
What is the difference between qualitative and quantitative observations?
Qualitative observations do not use numbers, quantitative observations do
What is this a picture of?

Hindenburg
This is the chemical symbol for a transition metal, derived from the Latin word aurum, meaning "shining dawn".
Gold = Au
What is this man's name

John Dalton
What TV show closely resembles Aristotle's theory?
Avatar: The Last Airbender