1) Bubbles forming
2) Energy released or absorbed
3) Formation of a new substance
4) Odor
5) difficult or impossible to reverse
What are signs of a chemical change?
number of atoms in H2O
The oxidation of iron (aka rust) is represented by the equation:
4Fe + 3O2 --> ????
How many atoms must be on the other side of the equation?
What is 10 atoms?
CO2 + H2O → C6H12O6 + O2
5g 10g 8g ?
What is the mass of the product O2?
7g or 7 grams
Reactants
Boiling water or melting an ice cube is an example of this.
What is a physical change?
number of atoms in glucose, C6H12O6
What is 24?
Which of the following equation is impossible?
a) 2H2 + O2 --> 2H2O
b) Na + Cl --> MgCl
c) CaO + CO2 --> CaCO3
What is b?
SiCl4 + H2O → H4SiO4 + HCl
12g ? 10g 10g
What is the mass of H2O?
Is H2O a reactant or a product?
What is 8 g or 8 grams?
What is a reactant?
This is what we call the ending substances of a chemical reaction. (It is the creation of NEW substances.)
Products
This image describes... 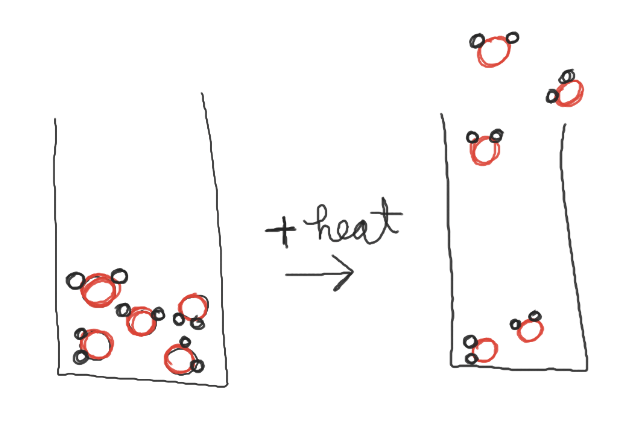
What is water evaporating or a physical change?
Number AND type of atoms in 2C8ClF15O
What is 50 total:
- 16 Carbon
- 2 Chlorine
- 30 Fluorine
- 2 Oxygen
If you react something on a scale, and the products measure more mass than the reactants, what must be true?
Hint: Think open/closed systems
What is "the system was open"
Exothermic or endothermic:
A bundle of sticks reacts with oxygen to create ash, carbon dioxide gas, and water.
What is exothermic, the bundle of sticks burned and released energy to the air around them
A chemical equation uses ______________ and ____________ to show how substances interact with each other and transform into new substances during a chemical reaction.
What is numbers and symbols?
This image shows...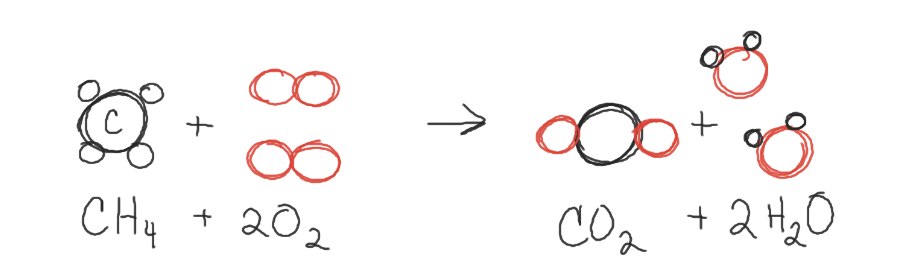
What is a chemical reaction?
What is something burning/combusting (the products are CO2 and water)?
Number and type of atoms in C8H(NO2)7
What is 30?
8 carbon, 1 H, 7N, 14O
What is always true about the mass of the products in a chemical reaction?
What is the mass always equal the mass of the reactants.
Why is photosynthesis endothermic?
When you light a candle, physical and chemical changes take place. List one of each.
Wax melting = physical
Wick burning = chemical
A student is trying to determine what a clear liquid is. She determines that its density is 0.789 g/cm3 and it is flammable.Which test that she performed took advantage of this liquid's physical properties?
What is density?
number of atoms in [Cr(H2O)4Cl2]Cl 2(H2O)
What is 22?
1 Cr, 12 H, 6O, 3Cl
Cassidy filled a flask with 5 grams of water, and left it on a scale. A day later the scale read three grams. How could this be true?
What is an example of an exothermic chemical reaction that is happening in every cell of your body right now?
What is cellular respiration?
Using elements in the periodic table, create a molecule (chemical formula) that represents a substance that has:
1. 3 different types of elements
2. A coefficient
3. Shows at least 2 of the elements with a subscript
Examples:
2 Na2Cl4O Coefficient is 2, Na=2, Cl=4
4 Fe3O3H Coefficient is 4, Fe=3, O=3
6 C6H12O6 Coefficient is 6, C=6, H=12, o=6