The substance in this density column with the lowest density.
Lamp Oil
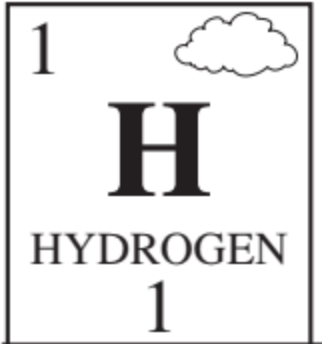
Draw the Bohr model that represents this element
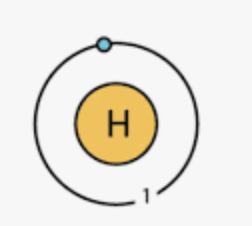
What is the charge for each of the subatomic particles?
Protons ________
Neutrons ________
Electrons ________
Proton = Positive (+)
Neutron = neutral (0)
Electron = Negative (-)

What is the atomic number for this element?
47
This image represents 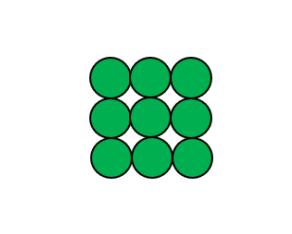
Element
The substance on this list that is NOT matter.
Blood, Helium, Air, Gravity, Lava, Table, Oxygen
Gravity
The element that is shown in this Bohr Model
Boron
The two subatomic particles found in the nucleus of an atom.
Protons & Neutrons

What is the Atomic Mass for this element?
108
This image represents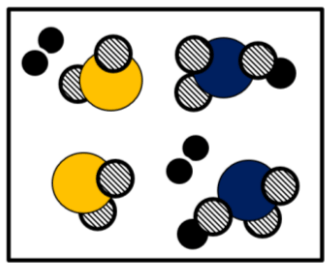
Mixture
Which of the following has the GREATEST volume?
B
Is the element shown in this Bohr Model.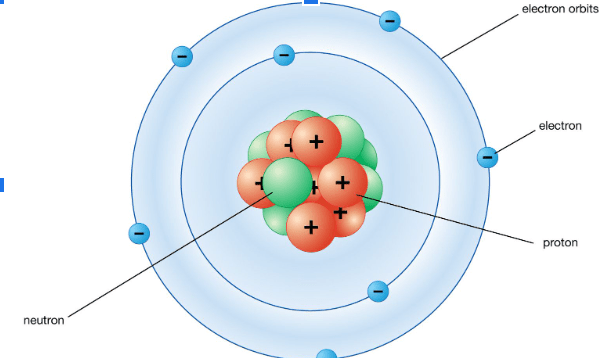
Nitrogen
The number of electrons is equal to
# of Protons
Chemical Symbol for Lead
Pb
 # of atoms
# of atoms
5
Which of the following has the LEAST amount of mass?
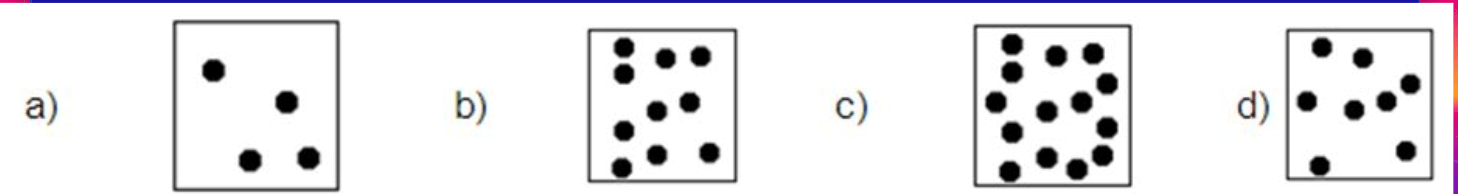
a
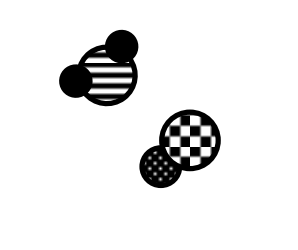
This image below shows two chemically bonded atoms. The two atoms are…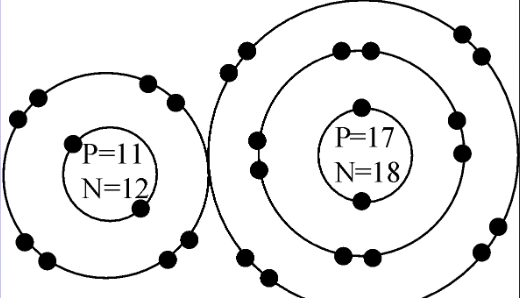
Sodium & Chlorine
An atom with 35 protons will have an atomic number of
35
The element found in Group 17 Period 5
Iodine
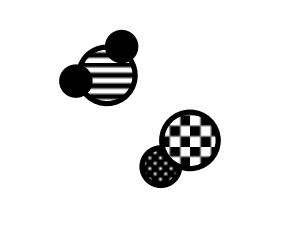
# of compounds
2
Sarah has an marble with a mass of 15g. After using the displacement method she finds that the volume is 5㎤. What is the DENSITY of the marble?
3 g/cm3
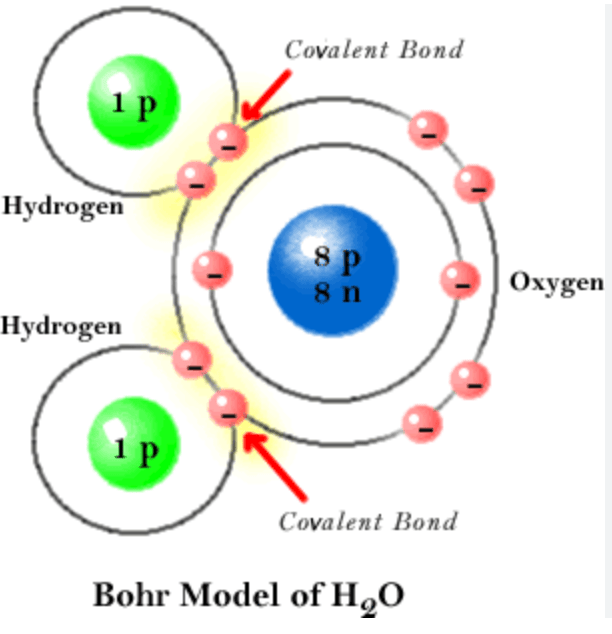
Draw a Bohr Model of H2O
The number of neutrons Beryllium has
5
This element has the same reactivity as Magnesium and is found in Period 4
Calcium
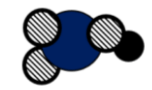
Atoms _______
Molecules _________
Elements _________
Compounds __________
Mixture Y or No
Atoms =5
Molecules = 1
Elements = 3
Compounds =1
Mixture =N