Name one Lab Safety procedure
Read lab instructions before starting, Always follow lab procedures exactly, Never do an unauthorized experiment, Never eat or drink in the lab, Never engage in practical jokes or horseplay, Wash hands after every lab.
Anything that has mass and takes up space is called
matter
A substance with no definite volume and no definite shape is a
gas
What is the atomic mass of Carbon (C)?
12.0107
Which parts of the atom move around outside the nucleus?
electrons
The step by step process of testing your hypothesis
experiment
The measure of the mass of a material in a given volume is called
Density
When a solid changes directly into a gas, this is called ______________.
sublimation
A column of elements in the periodic table is called
group
A subatomic particle that has a posative charge is called a(n)
Proton
A divided circle where each “piece” represents a proportional fraction. Used to show how a part of something relates to the whole
Pie graph
Salt water, and Kool-Aid are examples of what type of mixture?
Homogenous mixture
Boyle’s law relates the pressure of a gas to its...
volume
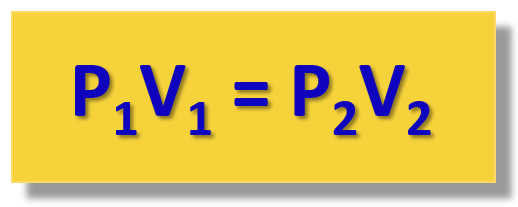
What family (group) does Argon (Ar) belong to?
Noble Gases (18)
In an atom, the second electron shell can hold up to how many electrons?
8
The metric prefix that means one thousand (1000) is...
kilo-
The law of conservation of mass states that during a chemical or physical change mass is
neither created or destroyed
Charles’ law relates the volume of a gas to its...
temperature
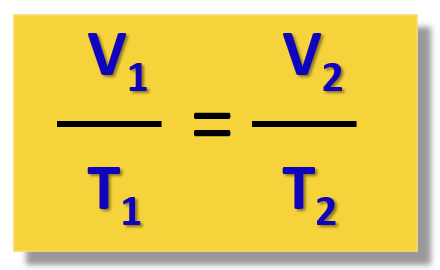
An iron atom has an atomic mass of 56. Its atomic number is 26. How many neutrons does an atom of iron have?
30
Who used the word “atomos,” meaning cannot be divided, to describe the atom?
Democritus
Correct placement of variables on a graph
x-axis is...
y-axis is...
x-axis is the Independent variable
y-axis is the dependent variable
The ability of a substance to react with oxygen is an example of a ________________ property.
chemical
What is the density of a sample of liquid that has a volume of 125 mL and mass of 200g?

1.6 g/mL
Two different isotopes of an element have different
number of neutrons
How many atoms are in a diatomic molecule?
2