For each statement below, determine if it describes a solid, liquid or gas.
1. No definite shape but definite volume (medium amount of energy)
2. Definite shape AND volume (low energy)
3. No definite shape or volume (high energy)
1. Liquid
2. Solid
3. Gas
Compounds are ________ or more atoms of different elements that are __________ bonded together.
2, chemically
Atomic Mass is equal to this
What is protons + neutrons?
The law of conservation of matter states that matter cannot be __________ or __________.
created, destroyed
True or False:
There is definitely nothing new to discover about atoms.
False
Theoretical temperature where all particle motion stops, the absence of thermal energy.
Absolute Zero
Identify each list as either chemical or physical properties of matter.
List 1: Solubility, Malleability, State of Matter, Density, Melting point
List 2: Acidity, Basicity, Flammability, Reactivity
List 1: Physical
List 2: Chemical
Label each of the three images.
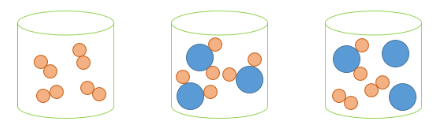
A B C
A - Element
B - Compound
C - Mixture
Identify each list of properties below as either properties of metals, nonmetals, or metalloids.
1.Brittle, dull, mostly gas, poor conductors.
2. High Luster (shiny), malleable, ductile, good conductors, most solid
3.Used in computer chips.
1. Nonmetals
2. Metals
3. Metalloids
Identify the type of bond:
1. When valence electrons are transferred (gained or lost). Between metal and nonmetal
2. When valence electrons are shared. Between two nonmetals
1. Ionic Bond
2. Covalent Bond
Particle 2 could either be a ___________ or a __________. Particle 1 must be a(n) _______________ because it is found outside the Nucleus.
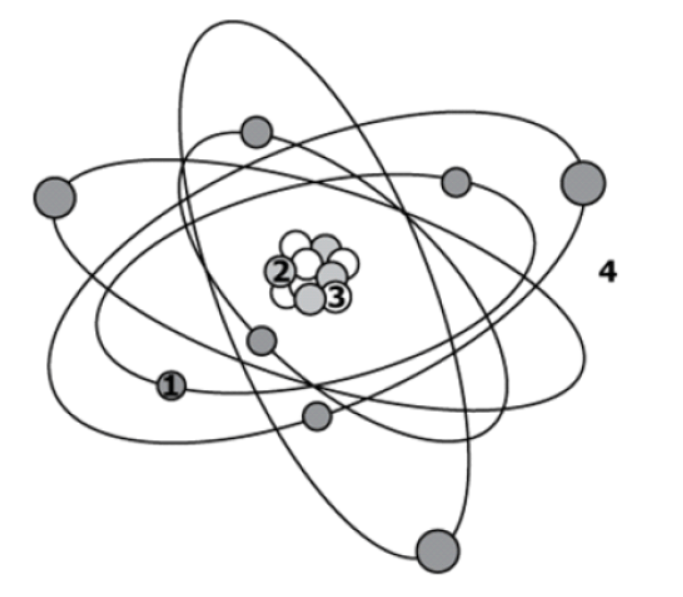
Proton, Neutron, Electron
Labels points A, C, and E
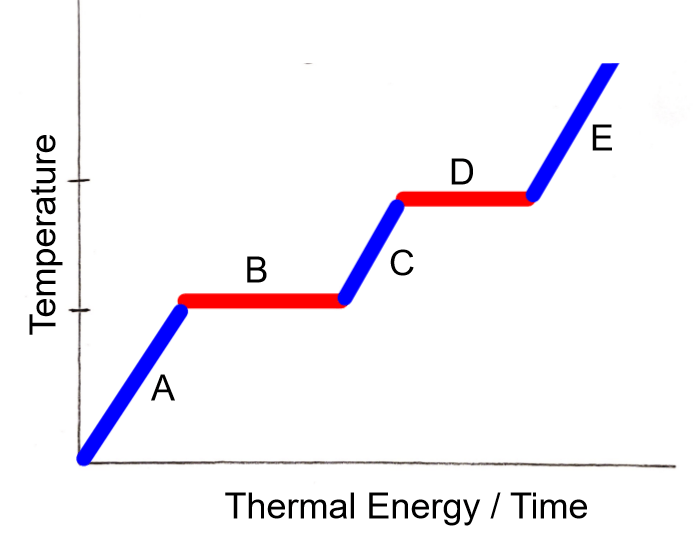
1. Solid
2. Liquid
3. Gas
______________ changes result in no new substance being created. Ex: cutting your hair
____________ changes result in a brand new substance being created Ex: burning paper
Chemical
Label image D and E.
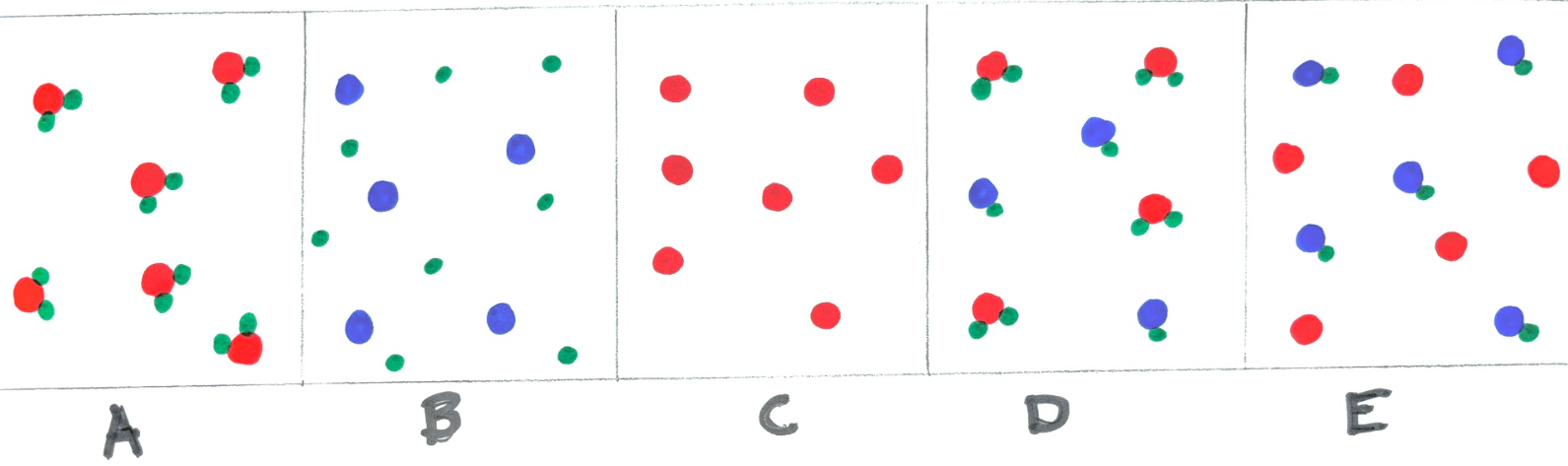
D - Mixture of Compounds
E - Mixture of Element and Compound
1. An atom with a positive or negative charge due to gaining or losing electrons
2. Two atoms of the same element with a different number of neutrons in the nucleus
1. Ions
2. Isotopes
2Na3PF4
The total number of atoms in the chemical formula.
16
Represents the probability or likelihood of an electron's location
The Electron Cloud
Identify each definition as either Conduction, Radiation, or Convection.
1.The transfer of thermal energy through direct contact
2.The transfer of thermal energy through currents in a liquid or a gas.
3.The transfer of thermal energy through space via electromagnetic waves
1. Conduction
2. Convection
3. Radiation
____________ properties can be observed by using your 5 sense or by taking measurements. You do not have to change the substance.
___________ properties can ONLY be observed by changing the substance.
Physical
Chemical
Label each statement as Element, Compound, or Mixture.
A: Two or more different elements chemically bonded
B: Made up Entirely of one type of Atom (all the same)
C: Two or more different substances in the same place but NOT chemically bonded
A: Compound
B: Element
C: Mixture
1. Elements in a period have the same number of this, but different properties
2. Elements in a Group/Family have similar these.
1. Energy levels or electron shells
2. Physical and Chemical Properties
Identify the bond as either Ionic or Covalent
Fr2S
Ionic
___________ (+) and ________ (neutral) are found in the nucleus of an atom, while ____________ (-) are found in the electron cloud surrounding the nucleus.
Protons
Neutrons
Electrons
1.ALLOW heat to easily flow through them, materials such as metals
2. DO NOT allow heat to easily flow through them, materials such as air and cotton
1. Conductors
2. Insulators
Identify each process as either a chemical or a physical change.
1. Dissolving Salt into Water to make Saltwater.
2. A plant preforming Photosynthesis.
1. Physical Change
2. Chemical Change
1. ___________ Mixtures look the same throughout, such as kool-aid.
2. In __________ Mixtures, there are clearly different components that make it up, such chex-mix.
1. Homogeneous
2. Heterogeneous
These two atoms are _________ of each other. (Same element)
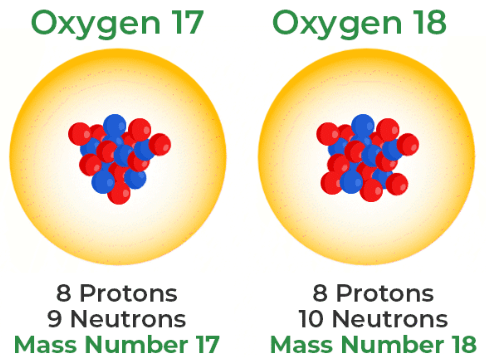
Isotopes
Identify the bond as either Ionic or Covalent
HCl
Covalent
1. These two subatomic particles have about the same mass and make up most of the atom's mass.
2.The smallest subatomic particle (almost 2000x smaller then the others)
1. Proton and Neutron
2. Electron
Identify each vocab term using the definition belows.
1.AVERAGE kinetic energy within a substance
2.The transfer of thermal energy
3.TOTAL kinetic energy within a substance
1. Temperature
2. Heat
3. Thermal Energy
We discussed 5 signs that a possible chemical change has occurred. List 3 of those signs.
Bubbling / Fizzing, Unintentional Color and/or Temperature Change, Smoke / Fire, Formation of a Solid (precipitate)
Label each as either an Element, Compound, or Mixture.
a - Calcium (Ca)
b - Nitric Acid (HNO3)
c - Air
d - Silicon (Si)
e - Baking Powder (NaHCO3)
a - Element
b - Compound
c - Mixture
d - Element
e - Compound
1. The only element to have 80 protons in it's nucleus
2. The number of Protons in an atom of Iron (Fe).
1. Mercury
2. 26
What are the missing coefficients?
____Fe + 4H20 ---> Fe3O4 + ____H2
3, 4
What are two reasons that the model of the atom has changed over time, demonstrating the Nature of Science?
1. Improved Technology
2. Collaboration among scientist
3. More detailed and Specific Research
All helped lead to new discoveries
Label Points B and D (could be two answers for each)

B - Melting or Freezing
D - Condensation or Boiling
Which two samples are made of the same substance?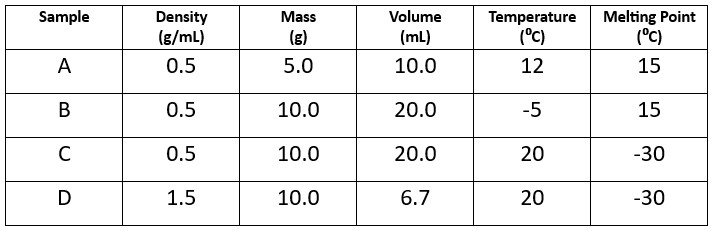
Sample A and Sample B
Mixtures can be separated by ______________ means whereas compounds can only be separated by __________ means.
Physical, Chemical
An atom of Magnesium has 12 protons, 12 neutrons, and 12 electrons. This is that Atom's Atomic Mass.
24
What are the missing coefficients?
____HCl + ____Na ---> ____NaCl + H2
2, 2, 2
Identify each of three statements below as either a Scientific Law, Theory, or Hypothesis
1. If I drink more water throughout the day, I can run further at practice.
2. The _________ of Gravity explains WHY an apple falls to the ground when dropped.
3. F = m*a
1. Hypothesis
2. Theory
3. Law
1. Thermal energy always moves from ______ substances to ______ substances.
2. Name the three methods of heat transfer.
2. Conduction, Convection, Radiation