A state of matter that has no fixed shape or volume
Gas
A _______ is a state of matter with a fixed volume but no fixedshape.
liquid
What is the definition for Element?
a substance that only contains one type of atom
A measure of the amount of matter present in a given volume of substance.
Density
Where are the metals located on the periodic table?
Left side
A student predicted that saltwater would freeze faster than freshwater. The data did not support this prediction. What should the student do next?
a) Assume the hypothesis was wrong and move on
b) Ask new questions about freezing points
c) Stop the experiment
d) Change the data
Ask new questions about freezing points
I have ph of 7?
Water
what is the difference between mass and weight?
mass is the amount of matter in an object
weight is the force of gravity acting on that object
A _______ is a state of matter with a fixed shape and volume.
solid
Pure substance or Mixture
Heterogeneous Mixture
Marcie wanted to find the density of a silver bracelet. She found that its mass was 21 grams and its volume was 2 cm3 . What was the density of the silver bracelet? Density = Mass/Volume.
A. 21 g/cm3
B. 10.5 g/cm3
C. 19 g/cm3
D. 2 g/cm3
B. 10.5 g/cm3
because...
Density = Mass/Volume
D= 21 g / 2 cm3 = 10.5g/cm3
Brittle, low density, dull and low melting points are characteristics of?
Non metals
Why do scientists repeat experiments conducted by others?
a) To check the reliability of the results
b) To change the outcome
c) To copy the experiment exactly
d) To find mistakes in the data
To check the reliability of the results
I donate h+ ions when dissolved in water?
Acids
A characteristic of a substance that can be observed or measured without changing its composition
A physical property!
What is the SI base unit for mass
kilograms [kg] or grams [g]
What type of substance would this be considered?
Compound
Density describes the relationship between ____ and ____.
mass and volume
I am a positively charged particle in the nucleus and am the atomic number of an element.
Proton
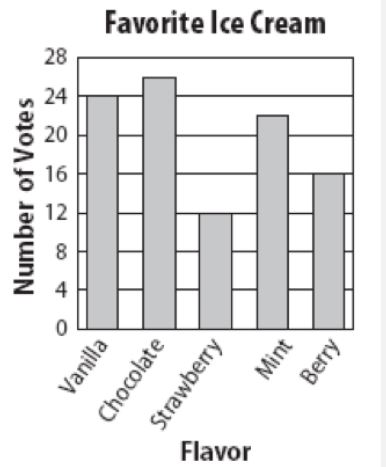
What is the independent variable?
Flavor
I don't turn red litmus paper blue and I don't turn blue litmus paper read, what am I?
neutral
The ability to rust, oxidize, burn, change ph, or be radioactive?
Chemical properties
Least energetic state of matter?
Solid
How many neutrons are in gold?
117
Hydrogen gas (H 2) can be found in trace amounts in Earth’s atmosphere. Which of these statements describes a physical property of hydrogen?
A. Hydrogen ions are found in acids.
B. Hydrogen gas is highly flammable.
C. Hydrogen ions react with oxygen ions.
D. Hydrogen gas is less dense than oxygen gas
D. Hydrogen gas is less dense than oxygen gas
because... this is the only statement describing a PHYSICAL PROPERTY
Name given to the semiconductors?
Metalloids
A student heats a beaker of water. She measures the amount of time it takes the water to increase in temperature by 5 degrees Celsius. What type of scientific investigation is the student conducting?
A. comparative study
B. theoretical prediction
C. hypothetical analysis
D. observation experiment
D. observation experiment
because they are actively manipulating a variable (heating the water) and observing the resulting change in temperature over time.
Which option below places the pH values in the correct order, from WEAKEST ACID to STRONGEST ACIDs?
Ductile, luster, malleable, smell, color are all...
physical properties
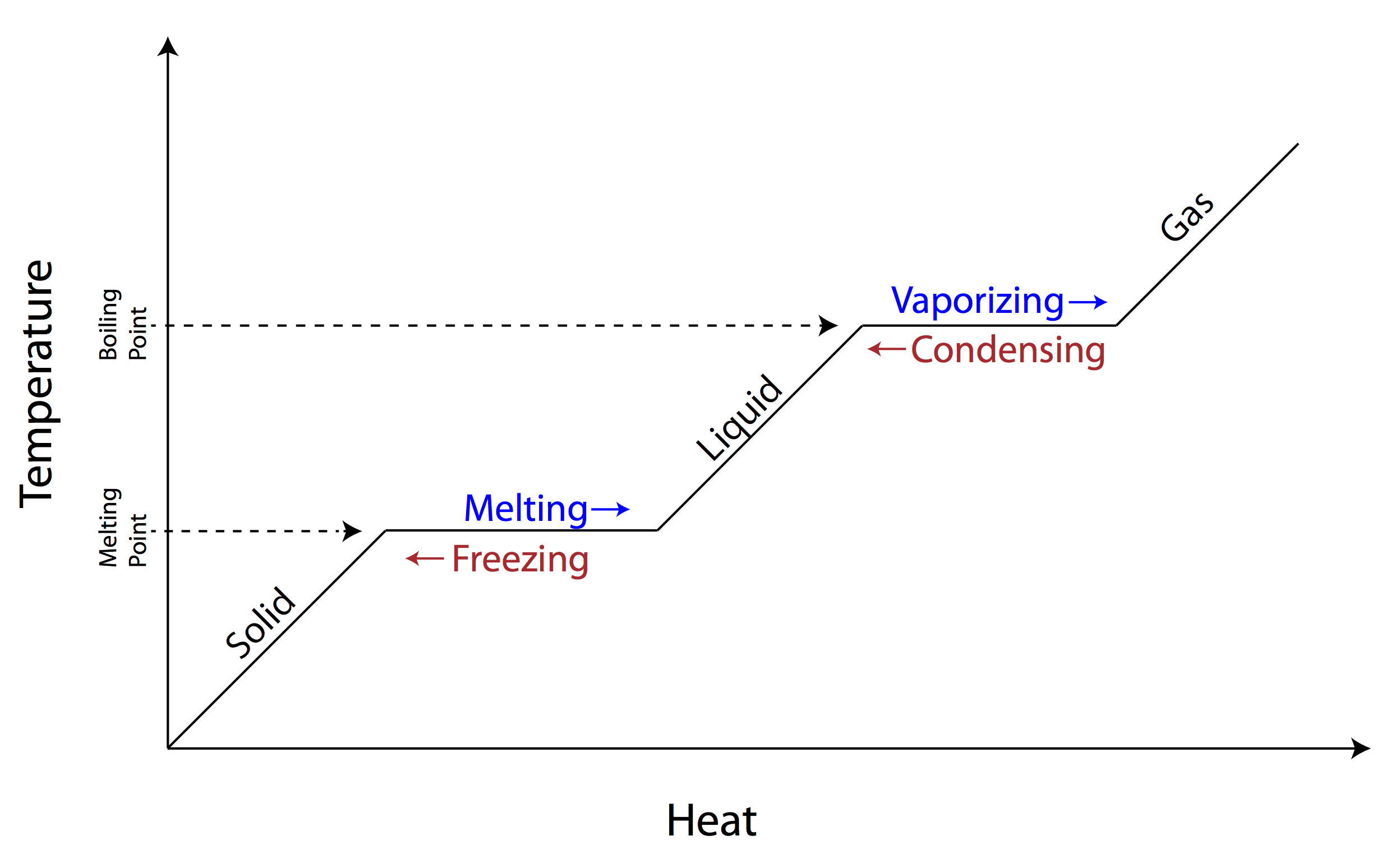 When a solid changes to a liquid?
When a solid changes to a liquid?
Melting
For a laboratory exercise in class, a student is given a mixture of small pieces of iron, sand, water, and salt. What physical property could be used to best separate the iron from the other ingredients?
A. solubility
B. magnetism
C. melting point
D. electrical conductivity
B. magnetism because... iron is magnetic, meaning it will be attracted to a magnet, allowing you to easily separate it from the other components in the mixture which are not magnetic.
Define volume
volume is the amount of space occupied by matter.
Least reactive elements on the periodic table are located in what family #?
18
Which of these is most needed for a study to be a valid scientific experiment?
A. results
B. observations
C. a control variable
D. a graph of the data
C. a control variable
because... to be considered reliable, as it allows for comparison between the experimental group and a group where the independent variable is not manipulated, ensuring the observed effects are truly due to the variable being tested
When mixed together an acid and base will neutralize and produce?
Salt