What is the top number on a square on the periodic table?
Atomic Number
Who created this model:

Niels Bohr
Everything in the world is either composed of ______ or ______.
Matter or Energy
What is the formula to finding density?
D = m/v
How is an Ion formed?
When an electron is given or taken
What is the bottom number on a square on the periodic table?
Atomic Mass
Who created this model:

JJ Thompson
Everything composed of matter is either _____ or a _______.
Pure or a Mixture
How do you find the volume of an irregular object?
Get a graduated cylinder, fill it up with water (taller than the object), place the object in the cylinder, subract the original number of milimeters from the number of millimeters after object is in water.
what is the most common ion for this element:
Negative or Positive
What are the letters in the middle of a square on the periodic table called?
Symbol
Who created this model:
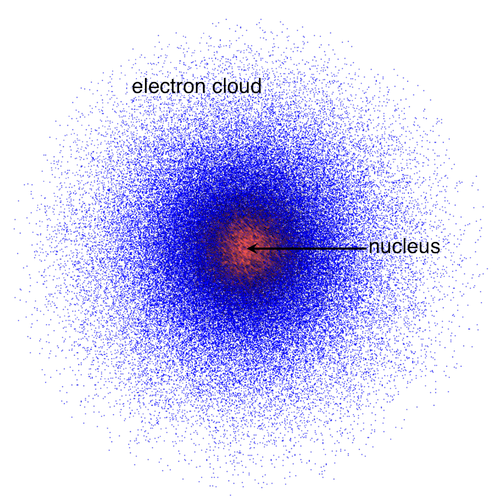
Schrodinger
Everything that is pure is a(n) __________.
Element
How do you find the volume of a regular object?
LxWxH
How do you read the periodic table?
Left - Right
Top - Bottom
Who created this model:
Rutherford
Everything that is a mixture is either _________ or ____________.
Homogeneous or Heterogeneous
How do you find the mass of a regular or irregular object?
A balance
What does the atomic number also represent?
Protons
What are valence electrons?
The number of electrons in the outer most energy level (max of 8).
Everything that is a mixture is always a _________ (and sometimes a __________).
Molecule (and sometimes a compound)
When mass increases and density decreases, it is called a(n) _________ __________.
When volume increases and density decreases, it is called a(n) _________ _________.
Direct Relationship
Inverse Relationship