a very small part or piece of something
Particles
a substance that is made up of 2 or more elements
Compound
a measure of how well a substance allows heat to pass through it
thermal conductivity
A substance that does not allow heat to pass easily
thermal insulator
a substance that can chemically react as an acid or as a base
amphoteric
the area of the atom surrounding the nucleus where electrons can be found
electron cloud
electrons found in the outermost energy level of an atom that are used in bonding
valence electrons
able to be shaped (as with a small mallet) without breaking
Malleable
evaporates easily
volatile
a material that is able to conduct electricity better than insulators but not as well as conductors
semiconductor
a particle which is smaller than an atom in size
subatomic particles
A researcher is using a particle accelerator in an experiment studying isotopes. How can the researcher change one isotope into a different isotope of the same element?
A. by removing valence electrons
B. by adding or removing neutrons
C. by adding valence electrons
B. by adding or removing neutrons
a measure of how well a substance allows electric current to flow through it
electrical conductivity
What trend does the reactivity of most nonmetals show in a periodic table, excluding the noble gases?
A. changes according to trends on the periodic table
B. decreases from left to right across the periodic table
C. increases from left to right across the periodic table
C. increases from left to right across the periodic table
Which element is most likely to be shiny?
A. boron (B)
B. calcium (Ca)
C. fluorine (F)
B. Calcium
Which statement best explains how the mass of an atom is organized?
A. Most of the mass is contained in the nucleus.
B. The mass is spread evenly throughout the electron cloud.
C. The mass is spread evenly throughout the whole atom.
D. Most of the mass is contained in the electron cloud.
A. Most of the mass is contained in the nucleus.
Which statement best explains how the mass of an atom is organized?
A. The mass is spread evenly throughout the electron cloud.
B. Most of the mass is contained in the nucleus.
C. Most of the mass is contained in the electron cloud.
B. Most of the mass is contained in the nucleus
Which properties make a metal a good material to use for electrical wires?
A. malleability and reactivity
B. ductility and malleability
C. conductivity and ductility
C. conductivity and ductility
Atom A and Atom B have the same number of protons and neutrons, but they do not have the same number of electrons.
Which statement describes the atoms?
A. The atoms have the same chemical symbol.
B. The atoms have different atomic numbers.
C. The atoms have different atomic masses.
A. The atoms have the same chemical symbol.
Which properties are characteristic of metalloids?
A. intermediate conductivity and a high melting point
B. low electrical conductivity and a low melting point
C. high electrical conductivity and a high melting point
A. intermediate conductivity and a high melting point
Which is a characteristic of the part of the atom marked "A”?
A. It contains most of the mass.
B. It is negatively charged.
C. It is mostly empty space.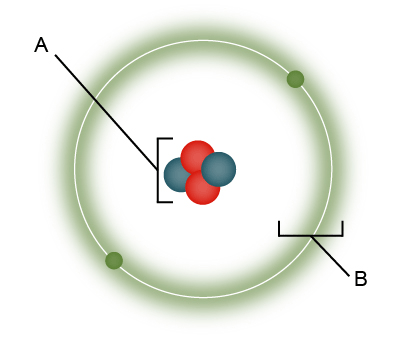
A. It contains most of the mass
Which statement describes a property of a proton?
A. is found outside the nucleus
B. has a positive charge
C. is repelled by electrons
B. Has a positive charge
The Venn diagram compares protons with electrons. Which shared property belongs in the region marked "B”?
A. Orbits the nucleus
B. Is found in the nucleus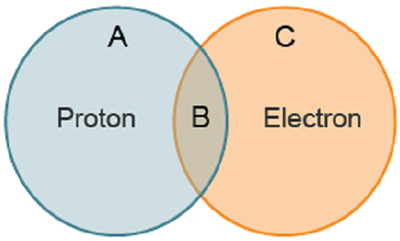
C. Is electrically charged
C. Is electrically charged
Scientists in a lab are working with two different samples of the element mercury. They know that the different samples are different isotopes.
Which property of the isotopes must be different?
A. the mass number
B. the atomic number
C. the electric charge
A. the mass number
Which lists the elements in order from most conductive to least conductive? (periodic table available)
A. potassium (K), germanium (Ge), selenium (Se)
B. germanium (Ge), potassium (K), selenium (Se)
C. selenium (Se), germanium (Ge), potassium (K)
A. potassium (K), germanium (Ge), selenium (Se)