The two subatomic particles that make up the nucleus of an atom.
What is the proton and neutron?
Pictured below:
What is an atom?
The scientist who contributed that a positive charge is concentrated in the nucleus of an atom, as pictured below.
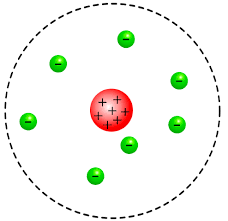
Who is Ernest Rutherford?
The charge of an electron.
What is negative?
All matter is made of atoms.
What is true?
Sodium has an atomic weight of 22.9898 and 12 protons, so it has ____ electrons, and _____ neutrons.
What is
12 electrons
11 neutrons?
The smallest unit of an element.
What is an atom?
The three subatomic particles that make up an atom.
What is the electron, proton, and neutron?
Pictured below, this image shows an unlabled...
What is the periodic table of elements?
The scientist responsible for the plum pudding model. He discovered atoms have negative particles.
Who is J.J. Tomson?
The charge of a proton.
What is positive?
The punchline to the meme below.
What is there was no reaction?
Chlorine has 17 protons, and 18 neutrons so it has ____ electrons, and roughly an atomic weight of ____.
What is
17 electrons
atomic weight of 35?
The wisdom from God or His creatures that makes things work in an orderly and beautiful way.
What is intelligence?
The answer to the image below.
What is hahaha?
Pictured in the image below, the number "4" indicates this.

What is the atomic number?
The scientist responsible for discovering electrons orbit the nucleus of an atom. He also developed the model as seen in the image below.
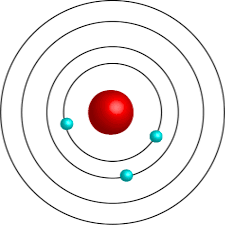
Who is Neils Bohr?
The charge of a neutron.
What is no charge?
An energy region in an atom where up to two electrons may reside are called circulars.
What is false, they are called shells or orbitals?
Aluminum has an atomic weight of 26.9815 and an atomic number of 13; therefore, Aluminum has ____ protons, ____ electrons, and _____ neutrons.
What is
13 protons
13 electrons
14 neutrons?
The variable used to quantify the amount of space an object takes up.
The three things the physical universe is made of.
What is matter, energy, and intelligence
Pictured in the image below, the number "9.0122" indicates this.

What is the atomic mass/weight?
The model, shown below, is the first model in the development of the atomic theory.

What is the Dalton model?
A property responsible for electricity and everything electrical in nature.
What is a charge?
The number of protons is the same as the number of neutrons in a stable atom.
What is false? Many times they are the same number, but not always. The number of protons is equal to the number of electrons.
The answer to this cringy meme.
What is ugh, nooooo, why...?
The variable used to quantify the amount of inertia in an object.
What is mass?
Mainly protons, electrons, and neutrons, although there are approximately 60 less commonly known of these.
Pictured in the image below, "Be" indicates this.

What is the element symbol?
The most current atomic model as pictured below.
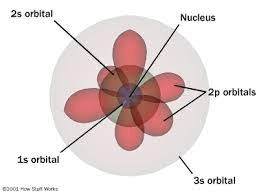
What is the quantum mechanical model?
An atom that has gained or lost one or more electrons, and has acquired a net electric charge (its charge has changed).
What is an ion?
The closer an electron is to the nucleus, the lower the energy AND the farther an electron is from the nucleus the higher the energy.
What is true?
Using the information from the element tile below, the number of protons is _____, electrons_____, and neutrons is ______.
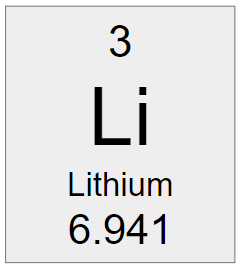
What is 3 protons, 3 electrons, and 4 neutrons?
Holds everything together and enables any process to happen.
What is energy?