Name this compound: H2SO4
Sulfuric Acid
What is the percent mass of Iron in Iron (III) Bromide?
18.89%
How many moles of Cl are in 22.5 Moles of FeCl2?
45 moles Cl
3.57 - 1.099 g O = 2.475 g C
1.099 x (1 mole/16 g O) = .0687 moles O
2.457 g x (1 mole/12.011g C) = 0.2061 moles C
.2061/ .0687 = 3 C
.0687/.0687 = 1 O
C3O
Rank these bonds in order of length and then separately in order of strength:
single
double
triple
length: triple < double < single
strength: single < double < triple
Name these compounds:
a. HClO2
b. NO2
a. Chlorous acid
b. Nitrogen dioxide
What is the percent composition of H and O in H2O?
H: 11.19%
O: 88.81%
How many grams of Chlorine are in 6.25 x 1025 moles of FeCl2?
7358.4 g
When an unknown compound is analyzed, it is found to have the following percentages of elements:
1.38 % O
32.89 % C
65.73 % H
Assume 100g of substance.
1.38g O
32.89g C
65.73g H
Convert to moles.
Divide all by smallest moles.
Numbers = subscripts.
HC2O3
Name these compounds:
a. FeS
b. BaO
c. HCN
d. HNO3
b. Barium oxide
c. Hydrocyanic acid
d. Nitric acid
Name these compounds:
a. KBr
b. H2S
c. FeCl3
a. Potassium Bromide
b. Hydrogen Sulfide
c. Iron (III) Chloride
What is the percentage of Oxygen in HNO3?
76.17%
How many atoms of Oxygen are in 55.6 grams of HSO4?
1.38 x 1024 atoms of H
Choose the 3 atoms out of the following six that are isoelectronic with each other:
C3-, P, S2-, Cl-, Ar, Se-
S2-, Cl-, Ar
All have 18 electrons.
Rank the four most electronegative atoms
1. F
2. O
3. Cl and N
Name these compounds:
a. HClO4
b. AgCl
c. MnS
d. HF
a. Perchloric acid
b. Silver chloride (Silver only has one possible charge!)
c. Manganese (II) Sulfide
d. Hydrofluoric Acid
What is the percent mass of carbon in acetate?
Acetate: C2H3O2-
32%
How many grams of Cl would combine with 24.4g Si to form SiCl4?
24.4 g Si x (1 mole Si / 28.086 g) x (1 mole SiCl4 / 1 mole Si) x (4 moles Cl / 1 mole SiCl4) x (35.453 g / 1 mole Cl)
123 g Cl
You are given 2.57 grams of a sample composed of tin and chlorine. If the mass of the tin alone is 1.17g, what is the empirical formula of the compound?
1. 2.57 -1.17 = 1.40g of Chlorine
2. Convert to moles
3. Divide by lowest mole number
SnCl4
Draw a Lewis Structure for CCl4
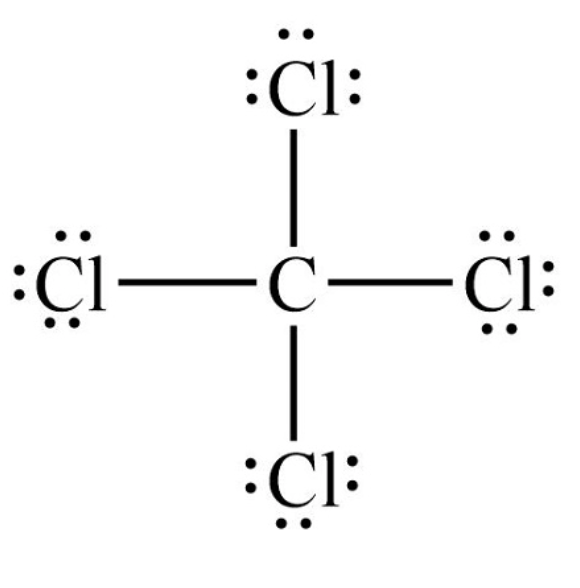
Name AND identify the type of this compound:
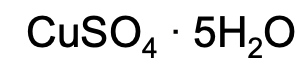
Copper (II) sulfate pentahydrate
What is the percent mass of iron in Iron (II) Bromide?
25.89%
Free Space: What is one thing you will do differently while studying for the second exam as opposed to what you did to prepare for the first exam?
:)
A white powder is analyzed to have 69.6% Ba, 6.09% C, and 24.3% O. What is its empirical formula?
1. Assume 100g
2. Convert all to moles
3. Divide all by smallest number of moles
BaCO3
What is the formal charge on Oxygen in H2O?
0
6 - 4 - 2 = 0