This man was the person responsible for the Billiard Ball Model
Who was John Dalton.
This is the number assigned to each number within the periodic table of elements that is also the number of PROTONS the element has
What is an atomic number?
This grouping includes hydrogen, oxygen, carbon etc.
What are non-metals
This is the name for the outermost electron shell for any given element/atom.
What is Valence.
This is the name for an element that has an abnormal amount of neutrons, making the atom unstable
What is an isotope.
This gentleman was the inventor of the solar system model.
Who was Hantaro Nagaoka
This is what we call a horizontal row in which elements are organized on the periodic table
What is a period?
This grouping is also known as 'semi-metals' or 'poor metals'.
What are metalloids.
A _______ has equal numbers of protons/electrons.
A _______ has more/less electrons than protons
An ATOM has equal numbers of protons/electrons.
A ION has more/less electrons than protons
How many protons does Darmstadtium have?
What is 110.
This was the gentleman responsible for discovering the NUCLEUS
Who is Ernest Rutherford
He invented what we have come to call the Periodic Table of Elements
Who is Dimitri Mendeleev?
This grouping of elements are all highly reactive and include only one electron in their valence shell?
What are alkali metals
How do we calculate the number of neutrons in an atom?
Atomic Mass - Protons = Neutrons
How many electrons fit into each of the first three shells of an atom?
2, 8, 8
This was the gentleman responsible for discovering the NEUTRON
Who is James Chadwick
This is the name given for each column on the periodic table.
What are families/groups?
What type of elements are all missing one electron from their valence shell?
What is Halogens
How many electrons are in an atom of Calcium?
What is 20.
Where are the protons, neutrons, and electrons in an atom?
Protons & Neutrons are in the NEUCLEUS
Electrons orbit in shells.
What do we call the model created by Erwin Schrödinger and Werner Karl Heisenberg?
What is the Quantum Model.
What element is found in period 6 and group 14?
What is lead (Pb)?
Which type of elements on the periodic table are known for being magnetic?
Transition Metals
What is 16.
32 - 16 = 16.
Who started using letters (instead of symbols) to represent/symbolize elements?
Who was Jöns Jacob Berzelius
This individual discovered subatomic particles and his model is known as the 'plum pudding' model (or raisin bun).
Who is J.J. Thomson
What element is found in period 4 and group 16?
What is Selenium.
What type of elements are generally non-reactive (give group number and name).
What is group 18, Noble gases.
Challenge: How many electrons are in an ION of Bromine?
Charge = -1
Electrons = 36
- 36 + 35 = -1
What element was represented by this symbol for Jupiter?
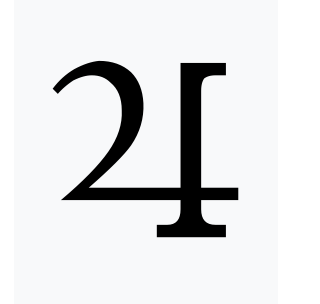
What is Tin.