As opposed to accuracy, this term refers to how close measurements of the same item are to each other.
What is Precision?
This is the element with the highest electronegativity.
What is Fluorine?
How many Oxygen atoms are there in 5Na(OH)3?
What is 15?
This is a substance that has been dissolved in water, dissociated into ions, and has the ability to conduct electricity.
What is an electrolyte?
This is energy that is transmitted in the form of electromagnetic waves.
What is radiation?
Hydro___-ic acids have an ion that ends in this.
What is -ide?
This type of physical property does not depend on the amount of matter present.
What is an intensive physical property?
The mathematical relationship of wavelength and frequency.
What is an inverse relationship?
The name of C7H14
What is Cycloheptane?
The definition of a weak acid.
A chemical formula that is written in lowest terms.
What is an empirical formula?
This is the person proposed the Billiard Ball Theory.
Who is John Dalton?
The amount of significant figures in 0000011100000.00000
What is 13?
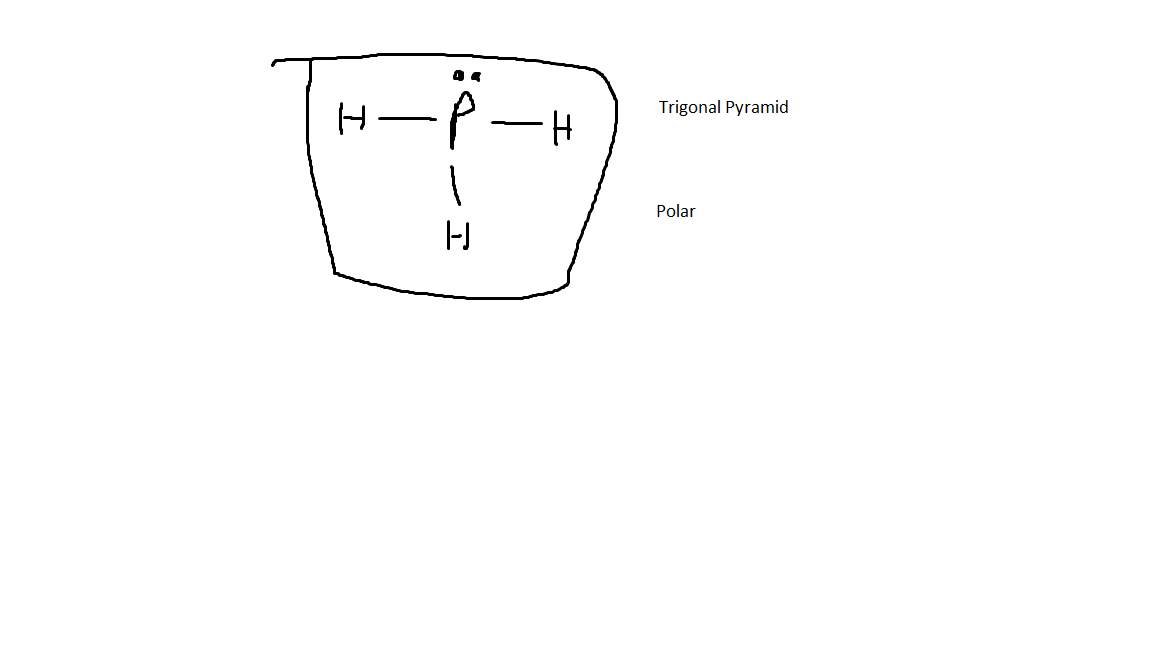
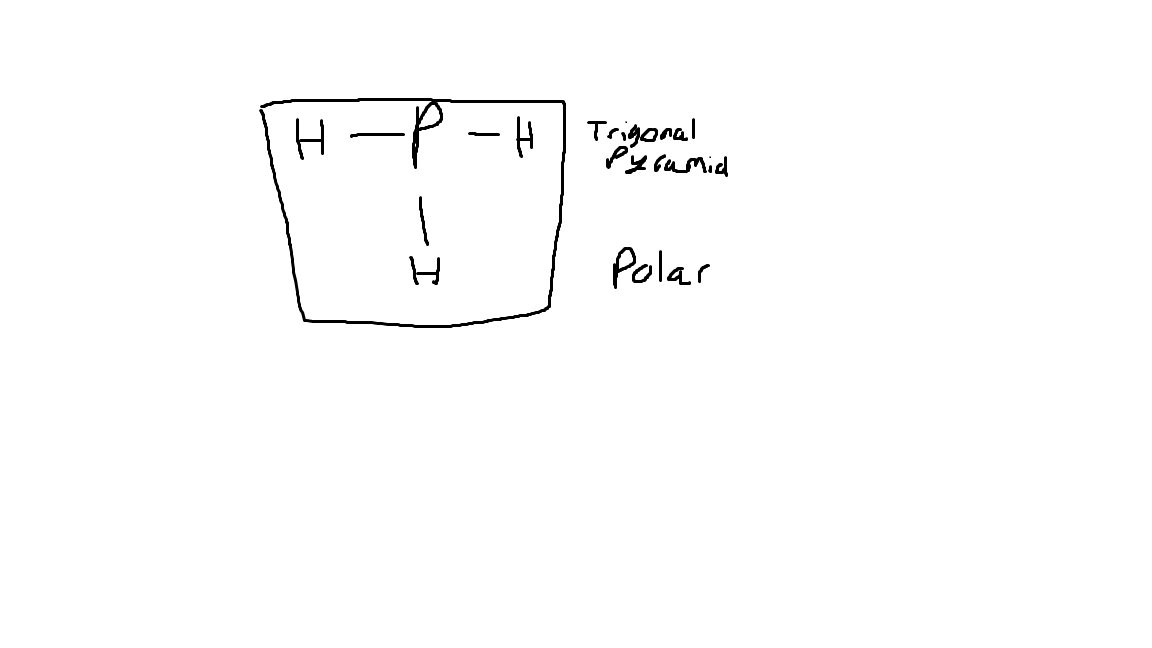
The Lewis Dot Structure for PH3, its VSEPR shape, and its polarity.
The chemical formula for Ammonium Phosphate.
What is (NH4)3PO4?
The hydroxide ion concentration of a solution with a pH of 1.2.
What is 1.6 x 10-13?
The percent composition of carbon in C6H12O6
What is 40%?
The equation for the combined gas law.
What is
(P_1V_1)/(n_1T_1)=(P_2V_2)/(n_2T_2)
The number of moles in 387.3 grams of Xenon
What is 2.950 moles?
Draw the Lewis Dot Structure for CO3-2 (Carbonate Ion).
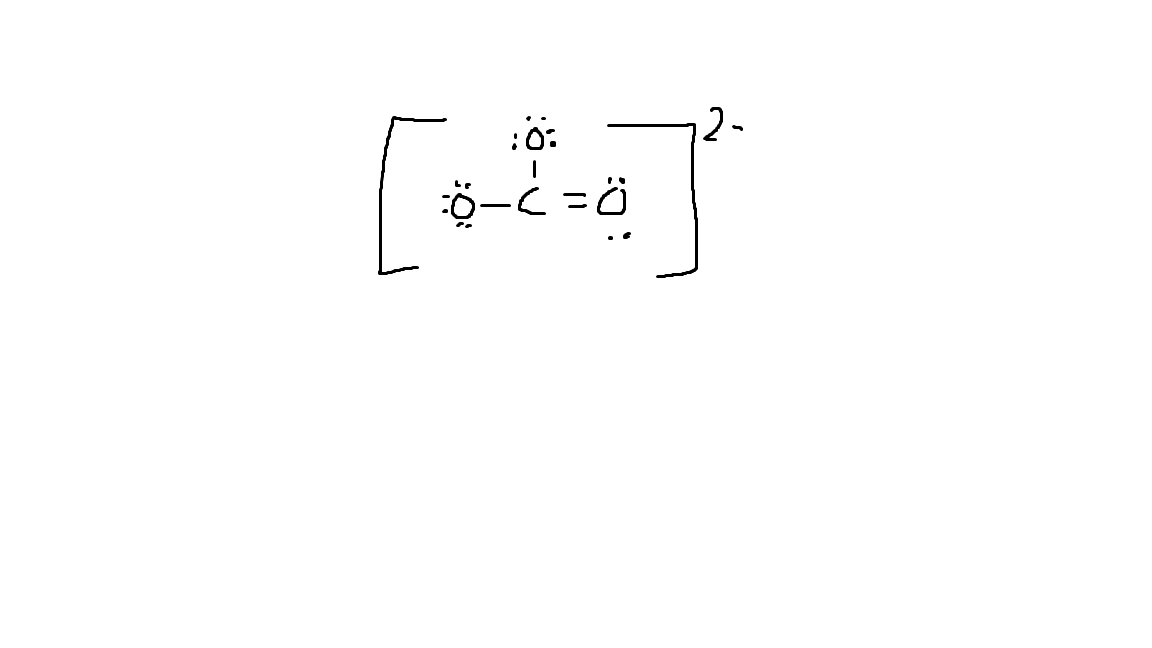
The balanced equation for when sodium reacts with bromine gas.
What is 2Na (s) + Br2 (g) = 2NaBr (s)
The net ionic equation for reaction of barium nitrate with sodium sulfate.
What is Ba2+(aq) + SO42-(aq) = BaSO4(s)
LiOH + KCl = LiCl + KOH
This is the percent yield of a reaction done with 20.0 grams of lithium hydroxide and excess potassium chloride, experimentally producing 29.0 grams of lithium chloride.
What is 81.92%?
These are the conditions of STP.
What are 1.0 atm and 0.0oC?
The equation for the Alpha Decay of Polonium-214.
What is (214 84 Po) = (4 2 He) + (210 82 Pb)?
The Element with the electron configuration of [Xe]6s24f145d106p3
What is Bismuth?
The balanced equation for when hydrochloric acid is mixed with a solution of strontium hydroxide
What is Sr(OH)2 (aq) + 2HCl (aq) = SrCl2 (aq) + 2H2O (l)
The molarity of 25.0 mL of HCl that is titrated by 60.00 mL of a 0.0330 M solution of Ca(OH)2
What is .158 M HCl?
The heat change in joules of a 20 gram piece of metal at 139oC dropped into 89 grams of water at 23oC. The temperature of the water rose to 36oC.
What is -4,840 J?
This is the new temperature of a gas in celcius that expanded from 15 cm3 to 50 cm3 originally at 12oC at a pressure of 1 atm.
What is 677oC?
Baby don't hurt me, don't hurt me, no more.
What is Love?