Periodic Table
Atomic Structure
Bonding
Quantitative Chemistry
Chemical Change
100
How are the elements in the periodic table arranged?
By atomic number
100
Where are electrons located in an atom?
In the shells/orbits circling the nucleus.
100
What is the difference between ionic and covalent bonding?
Ionic bonding - between a metal and nonmetal, metals gives electron(s) to nonmetal
Covalent bonding - between nonmetals, electrons are shared
100
What does the law of conservation of mass state?
The mass of the products in a chemical reaction must equal the mass of the reactants.
100
What is the difference between an Oxidation reaction and a Reduction reaction?
Oxidation - oxygen is gained
Reduction - oxygen is lost
200
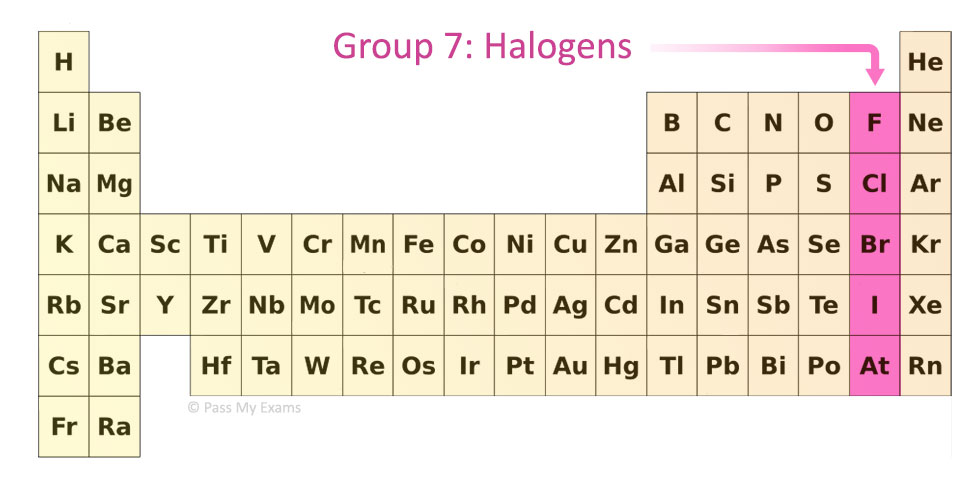
What is the name for group 7 in the periodic table?
Halogens
200
How many neutrons does magnesium have?
12
200
NaCl - ionic or covalent compound?
Ionic
200
Balance this equation:
____ Cu + ____ O2 → ____CuO
2 Cu + O2 → 2 CuO
200
What is the general equation for a neutralisation reaction?
Acid + Base → Water + Salt
300
What is the name for group 0 in the periodic table?

Noble Gases
300
Draw the atomic structure for sodium.

300
PH3 - ionic or covalent?
Covalent
300
Balance this equation:
____ H2O → ____ O2 + ____ H2
2 H2O → O2 + 2 H2
300
What is titration? Draw a diagram to show how it is carried out
A way to measure volumes precisely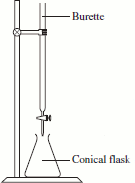

400
What is the name given to the elements in group 1?
Alkali Metals
400
What is an ion?
An atom with an electric charge due to the loss or gain of one or more electrons.
400
Draw how electrons would be transferred between Na and Cl.
400
Calculate the relative formula mass of CaCl2
111
400
What electrode do negative ions go to in electrolysis?
The positive electrode - anode
500
What do the elements in a group have in common with each other (in terms of electrons)?
Each element in a group has the same number of valence electrons (electrons in the outer orbit)
500
Draw the atomic structure for a fluorine ion.

500
Draw a dot and cross diagram for O2

500
Calculate the relative formula mass of H2SO4
98
500
List the 4 things that electrolysis needs in order to be carried out properly
1 - an electrolyte
2 - negative electrode (cathode)
3 - positive electrode (anode)
4 - a dc power supply