what is the law of conservation of mass?
what does this mean for a reaction that has 26 g of reactants? how many grams of products will be produced?
mass can neither be created nor destroyed
26g produced
What is the atomic number?
Number of protons
Convert 12 million into scientific notation.
1.2 x 10^7
What element has 17 protons?
Bonus: what charge is this element likely to take?
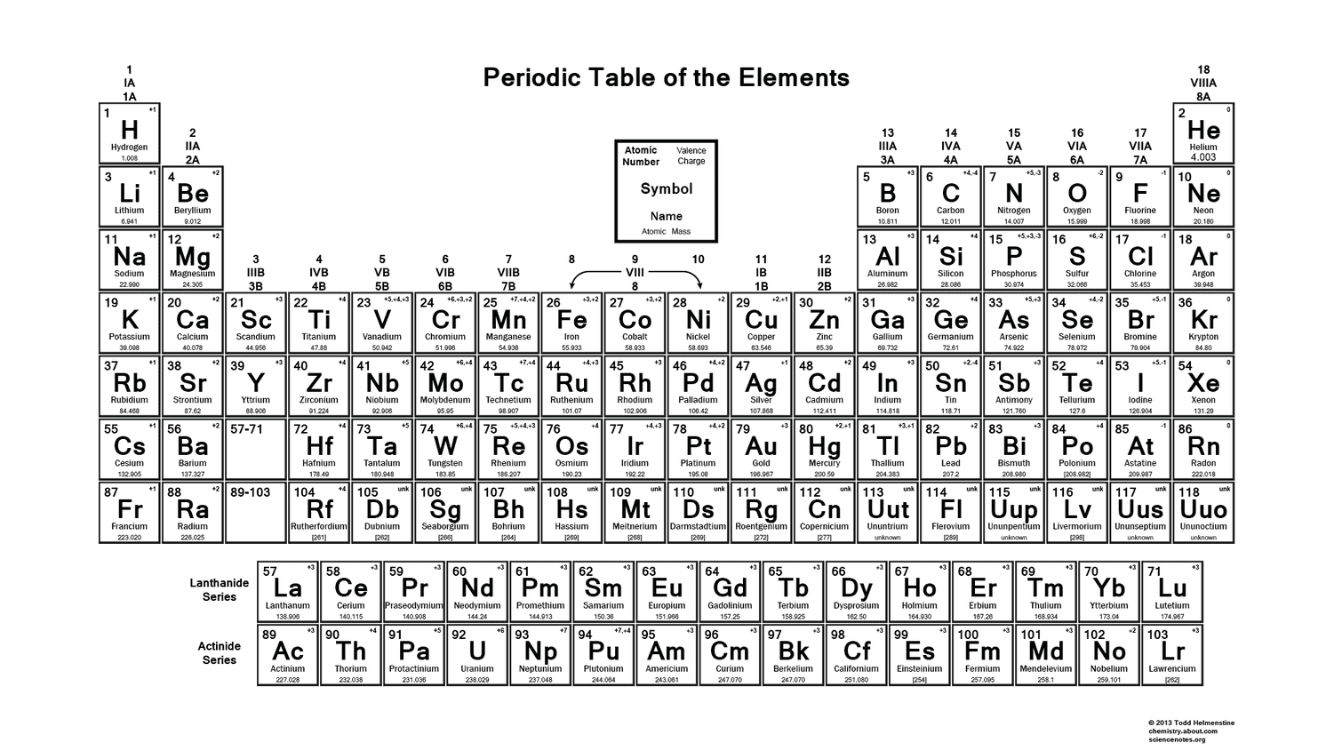
Chlorine
-1 charge
What is the difference between a chemical change and a physical change?
Chemical change = change in composition
physical change = change in physical properties (freezing / melting)
Who developed the atomic theory?
Early 1800s by Dalton
What is different between an isotope and the natural element? What changes?
Different number of neutrons. atomic mass changes but charge and element stay the same
Convert 674,322g into kg. Report with 3 significant figures.
674. kg
How is the periodic table organized?
What is a group, what is a period?
organized by chemical properties. atomic weight increases with each element.
period = row = across = get smaller across
groups = columns = down = similar chemical properties (noble gases, alkali metals, etc.)
How do the amounts of potential energy and kinetic energy change as atoms get closer and come in contact?
Farther away = higher potential energy
interact = higher kinetic energy
What did Rutherfords gold foil experiment prove? When was it performed?
1909
Proved that the atom is mostly empty with a tiny, dense, positively charged nucleus.
What is the difference between the mass number and atomic mass?
mass number: sum of protons and neutrons
atomic mass: average mass of atom, taking into account isotopes
You are given 150,000 mg of sugar. What is this in lbs? (2.2 lbs = 1kg)
0.33 lbs
Where are protons, neutrons, and electrons located?
Are electrons fixed in place?
Protons + neutrons in nucleus
electrons are not fixed in orbitals
Calculate the average atomic mass of Mg, to 4 sig figs.
Mg-24: 23.985 amu, 78.99%
Mg-25: 24.986 amu, 10.00%
Mg-26: 25.983 amu, 11.01%
(23.985 x 0.7899)+(24.986 x 0.10) + (25.983 x 0.1101) = 24.31amu
The Four postulates of the Atomic Theory (or in general, what is the atomic theory?)
1. elements are made of atoms
2. the atoms of each element are unique
3. atoms can join together in full number ratios to form compounds
4. atoms are unchanged in chemical reactions
What is the frequency of a light with a wavelength with 525 nm?
7.50x1014 Hz
Convert 263 degrees Celsius to Fahrenheit and Kelvin.
C = 5/9 (F-32)
F = 9/5C + 32
K = 273 + C
Kelvin = 536
Fahrenheit: 505
Name these groups on the periodic table: 1A, 2A, 7A, 8A
1A: alkali metals
2A: alkali earth metals
7A: halogens
8A: noble gases
Separate these terms into physical and chemical properties.
Flammability, malleability, acidity, reactivity, hardness, conductivity
Physical: malleability, hardness, conductivity
Chemical: flammability, acidity, reactivity
Place these occasions in order:
1. discovery of battery
2. Rutherfords gold experiment
3. discovery of electron
4. discovery of nucleus
1. discovery of battery (1800)
2. discovery of electron (1897)
3. discovery of nucleus (early 1900s)
4. rutherford gold experiment (1909)
Calculate the atomic mass of Silicon based on these 3 isotopes, with 5 sig figs.
Si-28: 92.23% 27.977amu
Si-29: 4.68% 28.976amu
Si-30: 3.09% 29.974amu
28.085 amu
If the density of a liquid is 10 g/mL, and you are given 87 mL of the liquid, what is the mass of the liquid in mg? Report with 2 significant figures and use scientific notation.
8.7 x 105 mg
Is this a cation or an anion?
-2, anion
What is the difference between a Bohr model and the Quantum mechanical model?
Bohr model: electrons are fixed in orbitals around the nucleus
Quantum mechanical:electron is treated as a wave - electron exists in space and cannot be pinpointed to a place.