Which phrase of the electron configuration for Mg is wrong? Write what it should be. (____ should be ____)
1s2 2s2 2p6 3s1
3s1 should be 3s2
What must the electrons do if there is one per orbital in the same sublevel? Use scientific term for credit!
(Orbital is one box. Sublevel is set of boxes)
Have the same spin
Bonus Question (500 pts): What's the name of the rule that says this?
True or False: Electrons can occupy the space between energy levels
False
Bonus Question (400 pts): What's this type of energy called and what analogy is used to describe it?
What is Group 18 of the Periodic Table called?
Noble Gases
Same number of valence electrons
Write out the electron configuration for Beryllium(Be)
1s2 2s2
What rule says that electrons fill the lowest energy level before going to the next? (Example: Fill 2s before going to 2p)
Aufbau Principle
Which part of the EM Spectrum is highest energy?
Gamma Rays
Which element is most similar to Silicon (Si)?
A. Aluminum (Al) B. Phosphorous (P)
C. Tin (Sn) D. Sulfur (S)
C. Tin (Sn)
Bonus Question (+200 pts): How do you know?
Which phrase of the electron configuration for Bromine (Br) is wrong? Write what it should be. (____ should be ____)
1s2 2s2 2p6 3s2 3p6 4s2 4d10 4p5
4d10 should be 3d10
Which phrase of the electron configuration is wrong? Write what it should be. (____ should be ____)
1s2 2s1 2p3
2s1 should be 2s2
Which rule says that in a sublevel, each orbital must have one electron before a second is added?
Hund's Rule
Fill in the blanks: _______ wavelength and ______ frequency = high energy
Short and High
Which element is in Group 2 and in the 5th period?
Strontium (Sr)
What is the main difference between Bohr's model of the atom and the Quantum Mechanical model?
In Bohr's model, electrons follow a defined path
Write out the electron configuration for Chlorine (Cl)
1s2 2s2 2p6 3s2 3p5
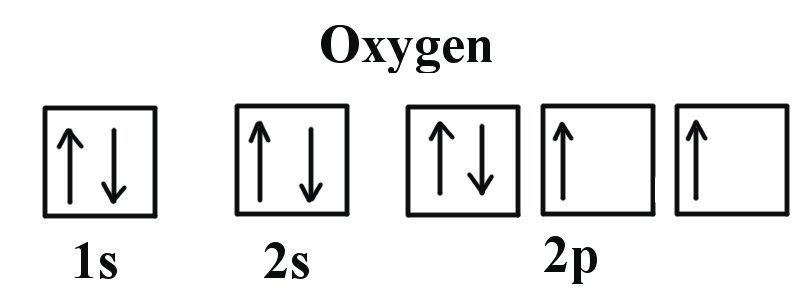
Draw the orbital diagram for Oxygen
What happens to wavelength if you increase the frequency?
Decreases
What are the green elements called?
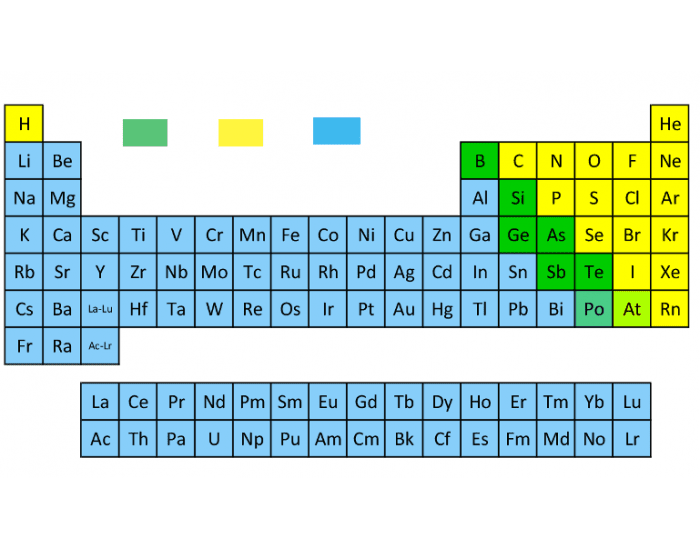
Metalloids
Bonus Question (300 pts): Are metals to the left or right of metalloids?
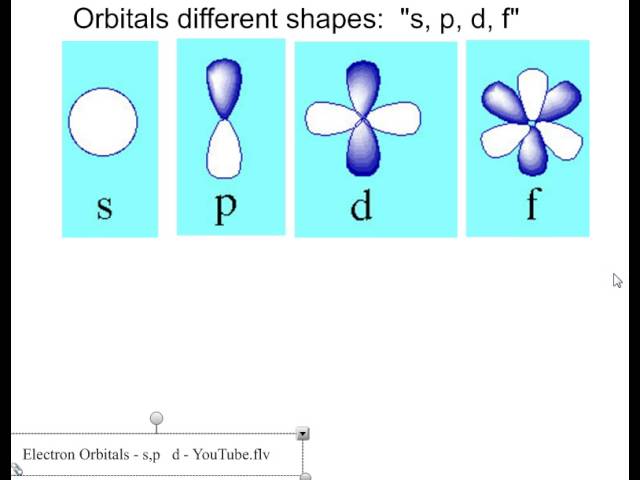
Draw the shapes for the s, p, and d orbitals
Write out the electron configuration for Manganese (Mn)
1s2 2s2 2p6 3s2 3p6 4s2 3d5
What rule was broken? (Assume 1s was filled)
Hund's Rule
How is color produced in the flame test?
Excited electrons jump back down to the ground state and emit colored light.
What sublevel is shown in green?
f sublevel
Bonus Question (200 pts): What must be done to the energy level when you enter the f sublevel?
What rule was broken in this orbital diagram?
Aufbau Principle
