(cont'd)
The conjugate base of CH3OH
CH3O-
The Bronsted acid that gives the weakest conjugate base:
HF, H2S, HCl, H2O
HCl
Predict whether Zn(NO3)2 will form a basic, acidic, or neutral salt solution.
acidic
What is the pH of a 0.75M HC2H3O2 solution?
Ka= 1.8 x 10-5
pH = 2.43
Calculate pH for 0.0080 M NH3 solution
Kb= 1.8 x 10-5
10.6
The conjugate acid of N3-
HN3
Rank the following compounds in order of increasing acidity:
CH3CH3, CH3OH, CH3NH2, CH3SH
CH3CH3 < CH3NH2 < CH3OH < CH3SH
Write the equation for the ionization and Ka expression for the following Bronsted-Lowry weak acid:
HOClO
HOClO(aq) + H2O(l) <=> H3O+(aq) + OClO-(aq)
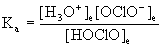
What is the pH of a 0.25M NH3 solution? Kb=1.8 x 10-5
pH=11.33
Calculate pH of a 0.25 M solution of KF
Ka for HF = 7.0 x 10-4
8.28
Given the reaction:
C6H5OH(l) + NH2-(aq) -> C6H5O-(aq) + NH3(aq)
This is the role "NH2-" plays in the reaction and its resulting conjugate...
What is a base that is involved in the conjugate acid formation of NH3?
Ka values for H2SO3, HNO2, CH3COOH, and HCN are respectively 1.3 x 10-2, 4 x 10-4, 1.8 x 10-5, and 4 x 10-10.
Given the above acids, this one produces the strongest conjugate base in aqueous solutions.
HCN
Determine if (NH4)2SO4 would form an acidic, neutral, or basic solution when dissolved in water.
Acidic
A solution is created by measuring 3.60 x 10-3 mol NaOH and 5.95 x 10-4 mol HCl into a container and then water is added until the final volume is 1.00 L. What is the pH of this solution?
11.48
What is the pH?
[H3O+] = 10-11
11
Given the reaction:
O2-(aq) + H2O (l) -> 2OH- (aq)
Associate each reactant/product with its appropriate label...
O2- = base
H2O = acid
2OH- = conjugate acid and conjugate base
Four acids along with their pKa are given:
Acid A: pKa 50
Acid B: pKa 15.7
Acid C: pKa 38
Acid D: pKa 3.2
This acid is NEXT to the most weakest out of the 4.
What is Acid C?
Predict if the solutions of the following salts are neutral, acidic or basic.
NaCl, KBr, NaCN, NH4NO3, NaNO2 and KF
NaCl, KBr = neutral
NH4NO3 = acidic
NaCN, NaNO2, and KF = basic
Calculate the pH of 0.025 M Ca(OH)2
12.7
What is the pH?
[OH-]= 10-8
6
Given the reaction:
SO3 + H2O <=> H2SO4
This reactant acts as a Lewis base.
H2O (lone pair of electrons is donated)
Identify the stronger acid:
HNO2 vs. HNO3
HNO3
Calculate the % ionization of a solution of 0.75M HF.
Ka = 7.2 x 10-4
9.6%
Calculate the pH of 0.05 M solution of HClO.
Ka= 3.5 x 10-8
4.39
My middle name?
Lee