Which substance is an Arrhenius acid?
A) HBr
B) NaBr
C) NaOH
D) NH3
A) HBr
Which positive ion must be present in an aqueous solution of an Arrhenius acid?
H+ or H3O+
Which statement describes characteristics of a 0.01 M KOH(aq) solution?
A) The solution is acidic with a pH less than 7.
B) The solution is acidic with a pH greater than 7.
C) The solution is basic with a pH less than 7.
D) The solution is basic with a pH greater than 7.
D) The solution is basic with a pH greater than 7.
What is the color of bromcresol green indicator in a solution with a pH value of 2.0?
yellow
Which substance always forms when an Arrhenius acid reacts with an Arrhenius base?
A) CO2
B) H2
C) CH3OH
D) H2O
D) H2O
Identify the only positive ion in the HC2H3O2(aq).
H+(aq) – H3O+ – hydrogen ions – hydronium
Which substance is an Arrhenius base?
A) HNO3
B) KNO3
C) LiOH
D) CH3COOH
C) LiOH
Which ion is the only negative ion produced by an Arrhenius base in water?
OH-
The [H3O+] of a solution is 1 × 10–8. This solution has a pH of
8
According to Reference Table M, what is the color of the indicator methyl orange in a solution that has a pH of 2?
red
Which solution reacts with LiOH(aq) to produce a salt and water?
A) KCl(aq)
B) CaO(aq)
C) NaOH(aq)
D) H2SO4(aq)
D) H2SO4(aq)
A sample of seawater has a pH of 8. Determine the new pH of the sample if the hydrogen ion concentration is increased by a factor of 1000.
5
Which formula can represent hydrogen ions in an aqueous solution?
A) OH–(aq)
B) Hg22+(aq)
C) H3O+(aq)
D) NH4+(aq)
C) H3O+(aq)
According to one acid-base theory, a water molecule acts as an acid when the molecule
A) donates an H+ ion
B) accepts an H+ ion
C) donates an OH– ion
D) accepts an OH– ion
A) donates an H+ ion
The pH of a solution is 8. When acid is added to the solution, the hydronium ion concentration becomes 100 times greater. What is the pH of the new solution?
6
Which indicator is blue in a solution that has a pH value of 7.0?
A) bromcresol green
B) methyl orange
C) phenolphthalein
D) thymol blue
A) bromcresol green
The reaction of an Arrhenius acid with an Arrhenius base produces water and
salt
Complete the equation below for the neutralization that occurs by writing a formula of the missing product.
2NaOH(aq) +H2SO4(aq) -->2H2O(l) + _________(aq)
Na2SO4
Which statement describes an electrolyte?
A) An electrolyte conducts an electric current as a solid and dissolves in water.
B) An electrolyte conducts an electric current as a solid and does not dissolve in water.
C) When an electrolyte dissolves in water, the resulting solution conducts an electric current.
D) When an electrolyte dissolves in water, the resulting solution does not conduct an electric current.
C) When an electrolyte dissolves in water, the resulting solution conducts an electric current.
What is the only positive ion found in H2SO4(aq)?
H+
The pH value of a solution is changed from 7.0 to 4.0. Describes the change in the hydronium ion concentration of the solution.
The hydronium ion concentration increases by 1000x
Based on the results of testing colorless solutions with indicators, which solution is most acidic?
A) a solution in which bromthymol blue is blue
B) a solution in which bromcresol green is blue
C) a solution in which phenolphthalein is pink
D) a solution in which methyl orange is red
D) a solution in which methyl orange is red
Which statement explains why 10.0 mL of a 0.50 M H2SO4(aq) solution exactly neutralizes 5.0 mL of a 2.0 M NaOH(aq) solution?
A) The moles of H+(aq) equal the moles of OH– (aq).
B) The moles of H2SO4(aq) equal the moles of NaOH(aq).
C) The moles of H2SO4(aq) are greater than the moles of NaOH(aq).
D) The moles of H+(aq) are greater than the moles of OH–(aq).
A) The moles of H+(aq) equal the moles of OH– (aq).
During a titration, 10.00 mL of acetic acid, HC2H3O2(aq), is completely neutralized by adding 12.50 mL of 0.64 M sodium hydroxide, NaOH(aq).
Determine the molarity of the acetic acid.
0.8M
Which formula represents an electrolyte?
A) H2O
B) CCl4
C) H2SO4
D) C6H12O6
C) H2SO4
Given :
NH3(g) + H2O(l) --> NH4+(aq) + OH–(aq)
In this system, the H2O() acts as
A) an acid, because it accepts an H+
B) an acid, because it donates an H+
C) a base, because it accepts an H+
D) a base, because it donates an H+
B) an acid, because it donates an H+
The pH of a 0.1 M solution is 11. What is the concentration of H3O+ ions, in moles per liter?
1 × 10–11

Which pH value is consistent with the indicator results shown in the table?
A) 1.0 B) 3.6 C) 4.5 D) 8.1
C) 4.5
Which salt is produced when sulfuric acid and calcium hydroxide react completely?
CaSO4
calcium sulfate
State how many times greater the hydronium ion concentration in the HCl(aq) is than the hydronium ion concentration in the HC2H3O2(aq).
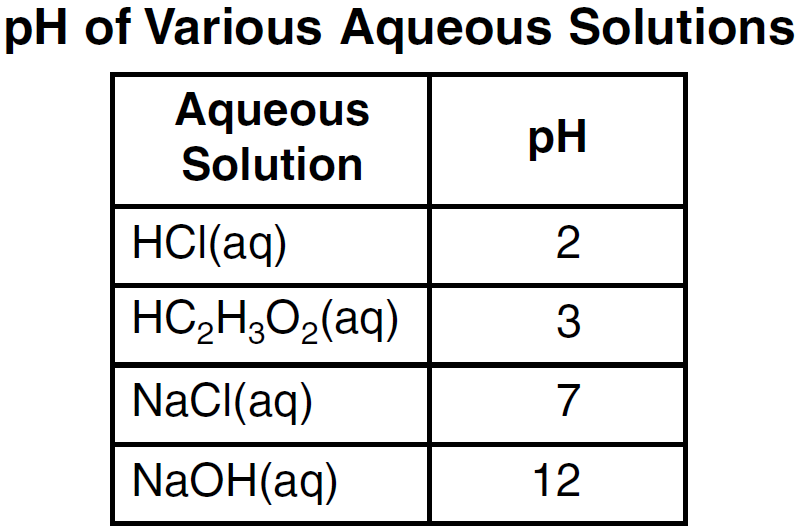
10x