What is one property of an acid?
Taste sour, electrolyte, & pH less than 7
What are some properties of a base?
Taste bitter, feels slippery, greater than 7, & are electrolytes
The pH scale ranges from _____ to _____.
0 - 14
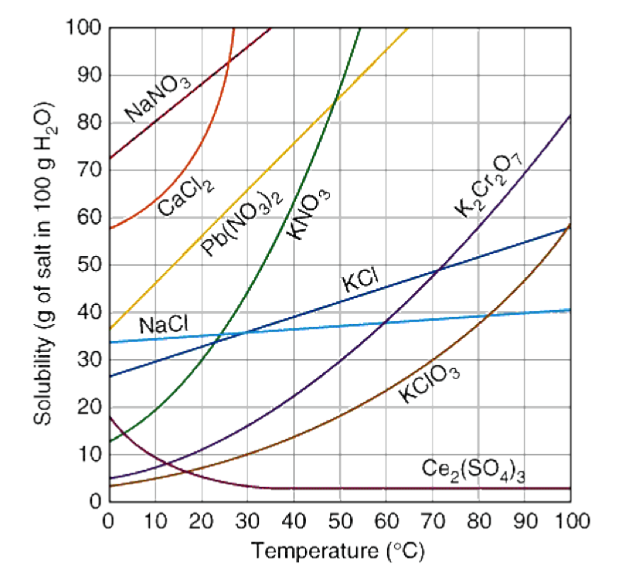
Which solute is least soluble at 10 ⁰C
KClO3
The ____________ is dissolved within the ___________ to make a Solution.
solute, solvent
Acids turn litmus paper what color?
Red
Bases turn litmus paper what color?
Blue
A chemical reaction between an acid & base is called what?
Neutralization Reaction

Which solute is the most soluble at 10 ⁰C?
NaNO3
What is the predominant relationship between temperature and solubility?
Positive, direct correlation
Acids have an excess of what ion?
H+ (hydrogen)
H3O+ (hydronium)
Proton
Bases have an excess of what ion?
OH- (hydroxide)
Acids and Bases... which ones conduct electricity?

How many grams of K2Cr2O7, are soluble in 100 g of water at 90 ºC?
70 g
Name three things that will increase the rate at which a solute dissolves in a solution.
Heat it
Crush it - smaller particles (more surface area)
Stir it
Which is the stronger acid: Wine or Lemon Juice?

Lemon Juice
Which is the weaker Base: Baking Soda or Bleach?

Baking Soda
Which of the following substances has the highest concentration of hydrogen (or hydronium) ions in solution?
Tomato Juice - pH 4
Vinegar

When 30 grams of KClO3 is dissolved in 100 grams of water at 60 ºC, the solution can be correctly described as what?
Unsaturated, Saturated, Supersaturated
Supersaturated
If a solution contains as much solute as a solvent can dissolve it is this.
Saturated
Name two solutions that, if mixed in equal quantities, would create the most acidic solution.
A and B
Name the two solutions that, if mixed in equal quantities, would create the most alkaline solution.
C and D
Name the two solutions solutions that, if mixed in equal quantities, would create a neutral solution
A and D

You have a solution of K2Cr2O7 containing 10 grams of solute at 50⁰C. How many additional grams of solute must be added to the solution to make the solution saturated?
20 g
What is the term that describes a solution that has more solute dissolved than it should be able to at that temperature?
Supersaturated
