Has a definite shape and definite volume
Solids
negative
In 3S4 the 3 represents this
Energy level (period)
These are used in bonding.
valence electrons
If there is a cation and anion (metal & nonmetal) use this method for writing formulas.
Criss cross charges
Gases
The charge of the nucleus.
positive
In 2p6 , the 6 represents this.
electrons
Bond with a sea of electrons
Metallic
Name for PCl4
Phosphorus tetrachloride
What is the correct measurement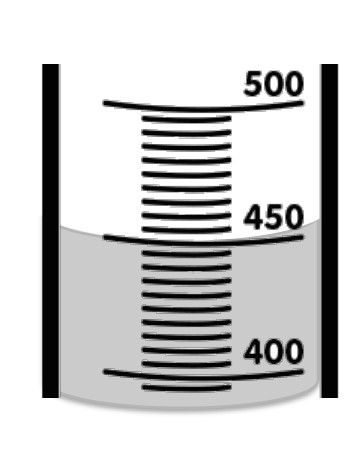
450mL
Isotope
The element represented by 1s2 2s2 2p6 3s2 3p6 4s2 3d6
Iron=Fe
Electrons in ionic bonds do this.
Transfer
The formula for sodium fluoride.
NaF
What is the mass?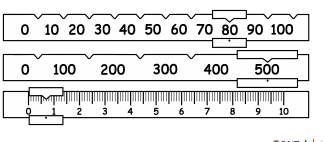
580.6g or 580.7g
The name of a particle that has gained or lost electrons.
ION
Extra for Cation and Anion
Cation=+ Anion=-
The noble gas that would be used in the electron configuration.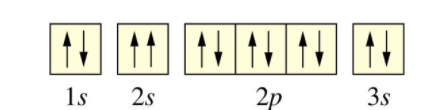
Neon
Types of elements that are anions.
non metals
Name for CaCO3
Calcium carbonate
Glass melting is an example of a __________ change.
physical change
Mass number for 
22
Electrons in their lowest energy state are in this.
ground state
A group of atoms covalently bonded (sharing electrons) that have a charge.
poly atomic ions
Elements that need a roman numeral in the name with 6 exceptions
transition metals
Extra for 6 that never have a roman numeral