What element has 1 proton?
Hydrogen
What subatomic particle has a positive charge?
protons
What element is modeled here?

Helium
Which group of the periodic table is known as the Noble Gases?
Group 18
What do we call an attractive force between two atoms that binds them together as a unit?
A bond!
What is element #10
Neon
What subatomic particle has a negative charge?
electrons
What element is modeled here?
Aluminum
What group of the periodic table has 1 valence electron?
Alkali metals
Which type of bond happens when electrons are shared between two atoms?
Covalent
What is element #20
Calcium
What subatomic particle has a neutral charge?
neutrons
What type of model is shown below for Lithium?
Lewis Dot Structure
Fluorine, Chlorine, Bromine, and Iodine make up part of group 17, the highly reactive _________ group.
Halogens
What type of bond occurs when an electron is transferred from one atom to another, resulting in a cation and an anion?
Ionic
What element has 17 protons?
Chlorine
Where in the atom can you find protons and neutrons?
the nucleus!
How many valence electrons does argon have? 
8
Why are noble gases unreactive?
They have a full outer shell or octet!
What type of bond is being depicted below?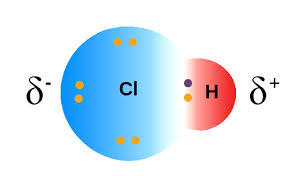
Polar covalent
What element has 5 protons?
Boron
How many electrons does the LARGEST element have?
118
What is the name of this handsome scientist who created the Bohr model?

Niels Bohr
What TWO groups of elements can be found at the bottom of the periodic table, separated from the main block?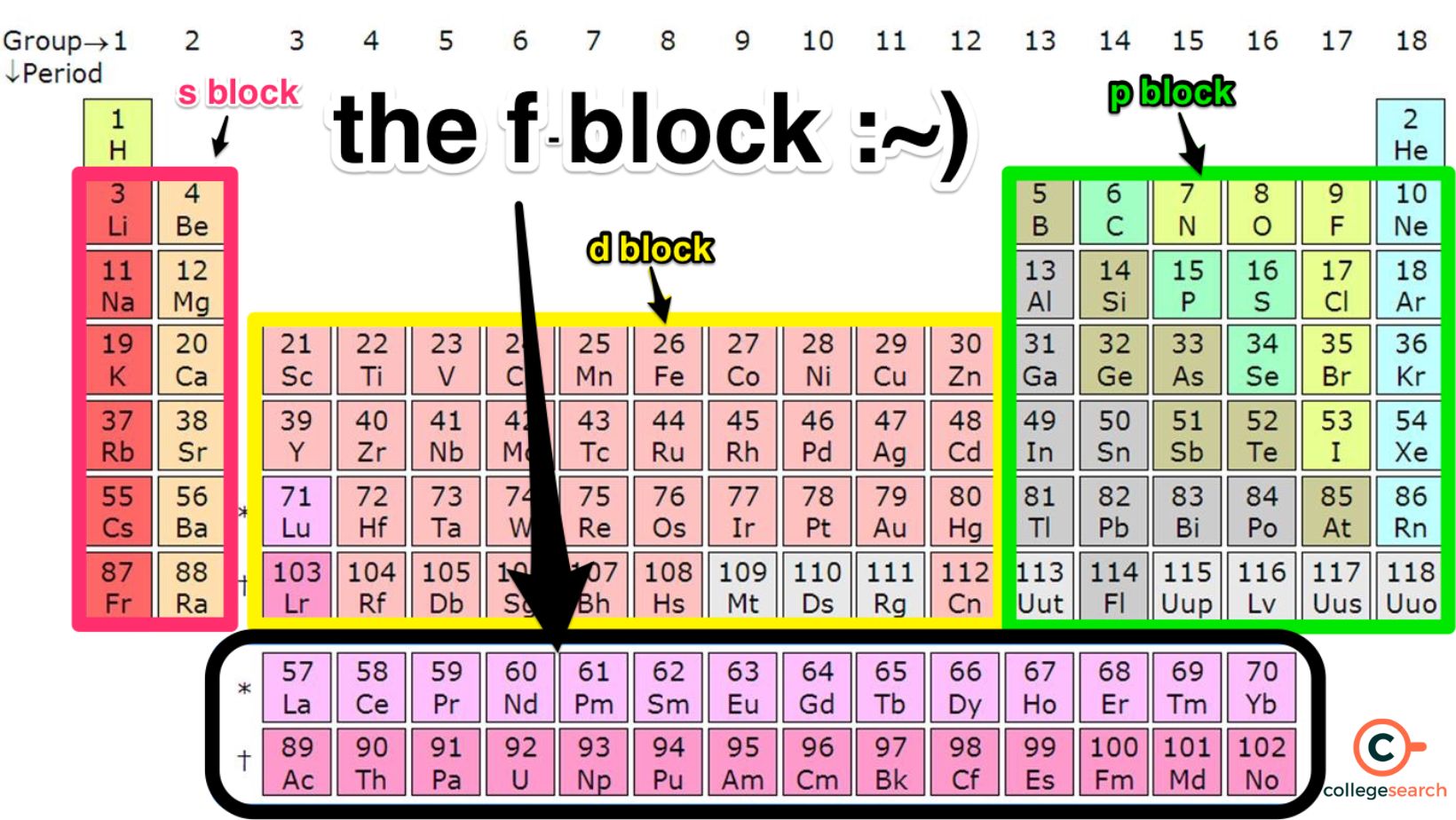
Lanthanides and actinides
What type of bond is depicted below?
Ionic
