What is the following molecule named?
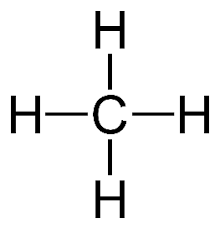
Methane
If the molecule has 1 Carbon, how many Hydrogens would it have?
4
What do all alkanes end with (last 3 letters)?
-ane
What is the general formula for Alkane?
CNH2n+2
What is a hydrocarbon?
A molecule made up of carbon and hydrogen
What is the name of the following?
Butane
If the molecule has 4 Carbon, how many Hydrogens would it have?
10
Are alkanes heavier or lighter than water?
Lighter
What type of bond does an alkane have?
Single-Bond
What bond does an alkene have?
Double bonds
What is the name of the following?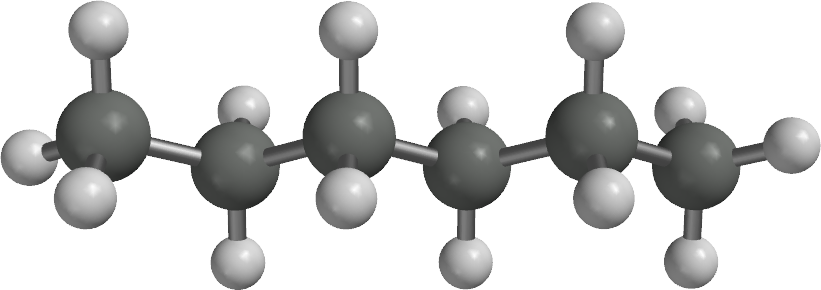
Hexane
If the molecule has 10 Carbon, how many Hydrogens would it have? *BONUS: what would it be called?*
Answer= 22
Bonus= Decane
Do Alkanes dissolve in water? If no, what do they dissolve in? If yes, how quickly do they dissolve?
No, they dissolve in non-polar solvents because they are nonpolar molecules.
What are the different groups of Alkanes?
The groups are linear straight-chain alkanes, branched alkanes, and cycloalkanes.
What bond defines an alkyne?
Triple bond
What is the name of the following?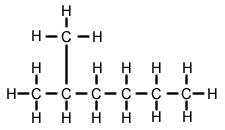
2-methyl hexane
How many Hydrogens does hexane have?
14
What colour are alkanes?
Colourless
Are alkanes saturated?
Yes
How many types of Aliphatic hydrocarbons are there? Bonus: What are the different types of Aliphatic hydrocarbons?
There are 3 types of Aliphatic hydrocarbons. Alkane, alkene, and alkynes
Name the following
4-ethyl-3-methylheptane
How many hydrogens does Octane have?
18
How do the boiling points of Alkanes change with chain size?
Bonus: how do the number of branches on alkanes affect boiling point?
As the carbon chain gets longer, there are more electrons in a molecule. This means that there are more (relatively) stronger intermolecular forces between the molecules. As a result, it takes more energy to break these forces, and thus the melting or boiling points increase.
Bonus: For isomers, the more branched the chain, the lower the boiling point tends to be.
How do alkanes benefit society?
They help heat our houses, move our cars, and drive our economy.
If there are over 26 Carbons, what is the boiling point of the alkane?
Over 400o C