The most reactive element
What is Fluorine?
moles of A / total moles in reaction
What is the equation to find the mole fraction of an equation?
Bond between H & a highly electronegative element (such as N,O, or F)
What is hydrogen bonding?
The concentrations are constant and the rates are the same
How to tell when a reaction is at equilibrum?
How chemical reactions take place in multiple steps
What is a reaction mechanism?
Law that states opposite charges attract
What is Coulomb's Law?
Metallic bonds are in this type of ocean
What is a sea of electrons?
How frequently a gas strikes the walls of its container
What is pressure?
k[A]n [B]m
What is the rate constant equation?
 Equivalence point roughly has a pH of 7
Equivalence point roughly has a pH of 7
What is a strong acid-strong base reaction?
Energy needed to remove an electron from an atom
What is ionization energy?
H2, N2, F2, O2, I2, Cl2, Br2
No attractive forces, mass of gas particles is negligible, collisions are all perfectly elastic, constant random motion
What is assumed in the Ideal Gas Law?
When a reaction ends with less energy then it started with
What is an exothermic reaction?
a solution with a high concentration of conjugate acid-base pairs
What is a buffer?
Used to convert moles to number of particles
What is Avagadro's number?
What is an interstitial alloy?
What is hard & brittle, with a high melting point, and only conducts electricity when melted?
can only be determined from experimental data
What are rate laws?
increase temperature, surface area, or concentration
How to increase the speed of a reaction?
multiply the relative abundance of an isotope by the mass of an isotope
How to find the average atomic mass of an isotope?
Bent, 109.5
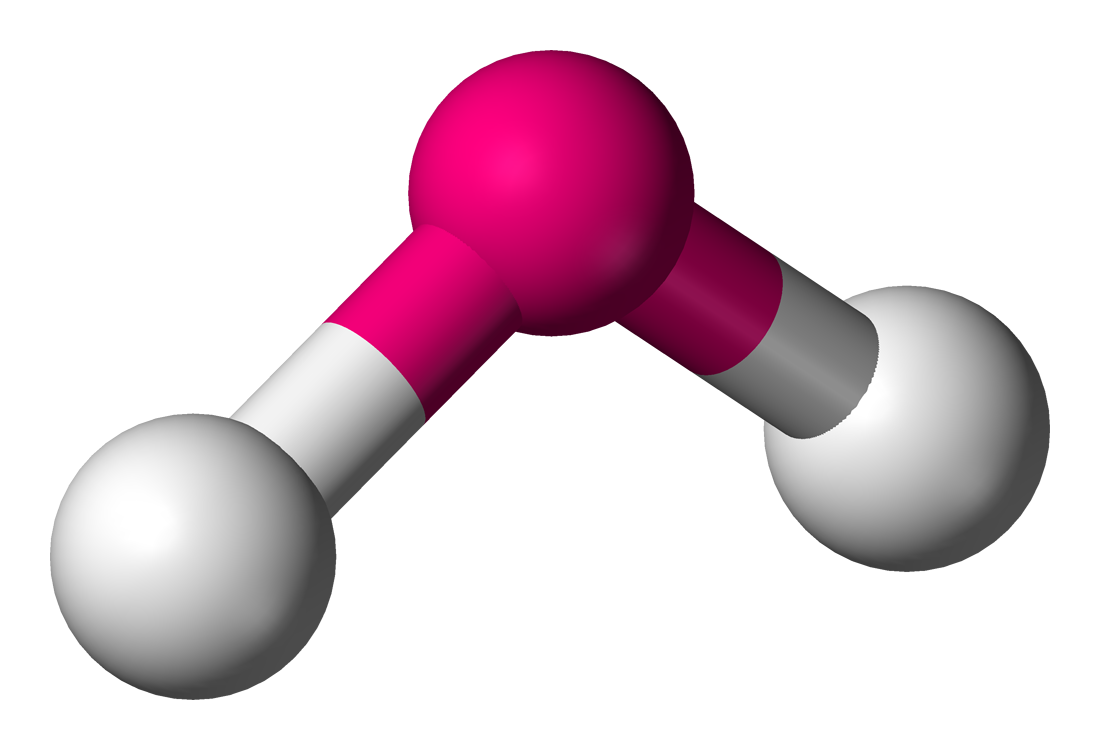
What is the molecular shape of water?
A non polar substance in a non polar mobile phase
What will move the farthest in a paper chromatography experiment?
ln[x] vs time
What is on the axes first order graph?
H2O2 + 2HI <--> H2O + I2
Solve for the Rate Equation
What is R = k[H2O2]1 [HI]2 ?