6.02 X 1023 particles or small furry animals
What is a mole?
or
What is a mol?
Type of bond in which electrons are shared
What is covalent?
Forces between particles
What are intermolecular forces?
Type of change in which a solid H2 turns into gaseous H2
What is physical?
Reaction in which
Delta H = -
What is exomthermic?
Even ionic sulfur has 16 of these.
What are protons?
Typoe of compound that can conduct electricity in liquid state or in solution?
What is ionic?
Weakest IMF
What are London Dispersion Forces?
What are LDFs?
What are Van der Waals?
What are dispersion forces?
Type of reaction in which a proton (H+) is transferred
What is an Acid Base Reaction?
On an energy diagram products minus reactants is also known as
What is
Delta H?
36 g of H2O is _______ mol
What is 2?
This type of substance has a "sea of electrons"
What is a metal?
The higher the boiling point the ......
What are stronger IMFs?
Type of reaction in which electrons are transferred?
What is REDOX?
or
What is an Oxidation Reduction Reaction?
When concentration has no effect on rate we say the reaction is ________
What is zeroth order?
What element is this?
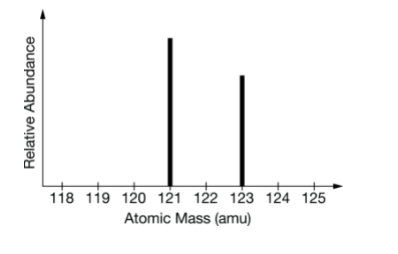
What is antimony?
or
What is Sb?
Rule that says each s and p block atom needs 8 electrons to complete its valence shell
What is the octet rule?
IMF that accounts for H2O having a higher BP than CH4
What is Hydrogen bonding?
What is dipole dipole?
Study of quantitative relationships in chemical reactions
What is stoichiometry?
Consumed in reactant step and produced in a later step......
What is a catalyst?
What element is this?
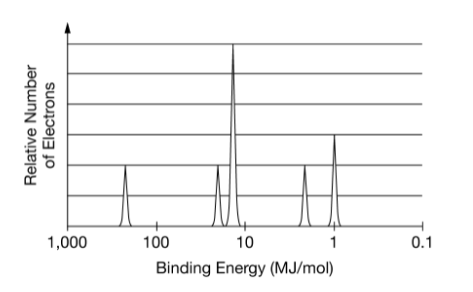
What is phosphorous?
or
What is P?
Bond angle for tetrahedral junctions
What is 109.5?
Molarity of 12 mol of NaCl in 500 mL of water
What is 24?
Method of determining unknown concentrations (especially of acids and bases)
What is a titration?
Increasing temperature increases this
What is rate?