1 mole = ______ molecules
What is 6.022 x 1022 molecules?

X2 = H2
Y2 = N2
O2 = Z2
Properties of ionic compounds:
What is brittle, not able to conduct electricity as a solid but can after melted into liquid?
Name some chemical changes:
What is burning, digesting, and rotting?
The order of this graph
What is the first order?
This will completely dissociate into ions in solution.
What is strong electrolytes?
The bond between SiO2.
What is covalent bond?
Name the qualities of solids, liquids, and gases:
Solids - rigid, fixed shape & volume
Liquids - not rigid, no fixed shape, fixed volume
Gases - not rigid, no fixed shape & volume
Name some physical changes:
What is phase change?
Type of reaction:
What is exothermic?
Name the element:
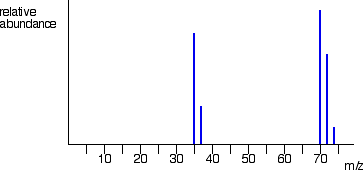
What is Chlorine?
This is the correct structure for NO3.
What is all of them?
(due to resonance and formal charges)
NaCL dissolved in water.

Write the Net Ionic Equation of this reaction:
AgNO3 + NaCl -> NaNO3 + AgCl
What is Ag+(aq) + Cl-(aq) -> AgCl(s) ?
Write the rate order: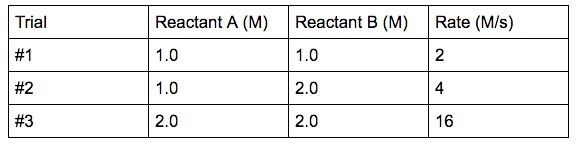
What is Rate = k [A]2[B]2
This element can replace Na+ in NaCl.
a) Ca
b) Mn
C) Rb
d) F
What is Rb?
This is the shape of GeO2 (both molecular geometry and electron geometry)
What is Linear?
Name the relationships between pressure, volume, and temperature:
Pressure and volume are inversely proportional.
Pressure/Volume are directly proportional to temperature.
Chemical change is to intramolecular change as physical change is to ______________
What is intermolecular change?
The higher temperature is this colored line:
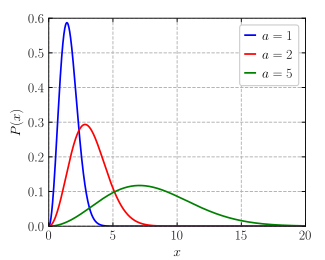
What is the green line?
Sr2+ has a smaller size than Br-
Why?
What is greater effective nuclear charge?
Name the hybridization between Carbon and Oxygen.
What is sp3?
List the colors from greatest to lowest wavelength.
What is Red, Orange, Yellow, Green, Blue, Indigo, Violet?
The limiting reagent in a chemical reaction is one that:
What is "is consumed completely"?
To deviate from the ideal gas behavior:
What is high pressures and low temperatures?