These reactions have a negative ΔH.
What are exothermic reactions?
This is the type of intermolecular force that two molecules of O_2 would experience.
What are London Dispersion Forces?
This substance has the following Lewis dot diagram:
What is H2O (water)?
The following chemical equation represents this type of reaction: HCl + NaOH to H2O + NaCL.
What is an acid-base reaction?
Peter, Cleavland, Quagmire, and Joe are known to frequent this establishment local to Quaghog, Rhode Island.
What is the drunken clam?
This is the amount of heat it takes to raise the temperature of an object by 1C or 1K.
What is heat capacity?
Molecules with a very low melting point are likely to experience a weak form of these forces.
What are intermolecular forces?
This substance has the following Lewis dot diagram

What is CCl_4?
An ionic bond is formed between these two types of elements.
What is a metal and nonmetal?
This dog belongs to Quahog resident Peter Griffin.
This is the amount of energy a 40g object, with a specific heat capacity of .13 J/(g °C), absorbs when being heated from 50 C to 70 C.
What is 104 J?
Between H_2 and O_2, this molecule has the highest boiling point.
What is O_2.
This is the Lewis Dot diagram for O_2.
What is: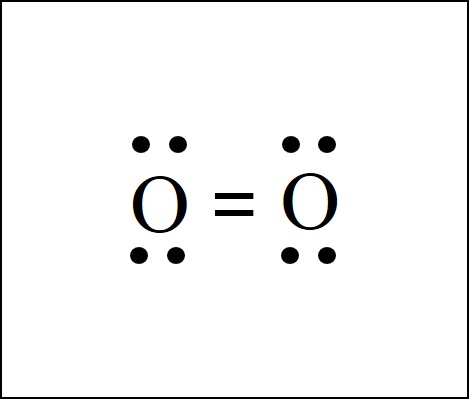
This type of reaction is shown in the following chemical equation: Al(s) + Cu2+(aq) --> Al3+(aq) + Cu(s).
What is a redox reaction?
This former teacher was known for frequently - and unintentionally - testing the fire alarm.
Who is Kriegs?
This type of reaction reaction has a positive Cell standard potential.
What is a thermodynamically favored reactions?
This misnomer for a type of intermolecular force refers to it as a "bond".
What are hydrogen bonds?
This element has the following Lewis dot diagram:
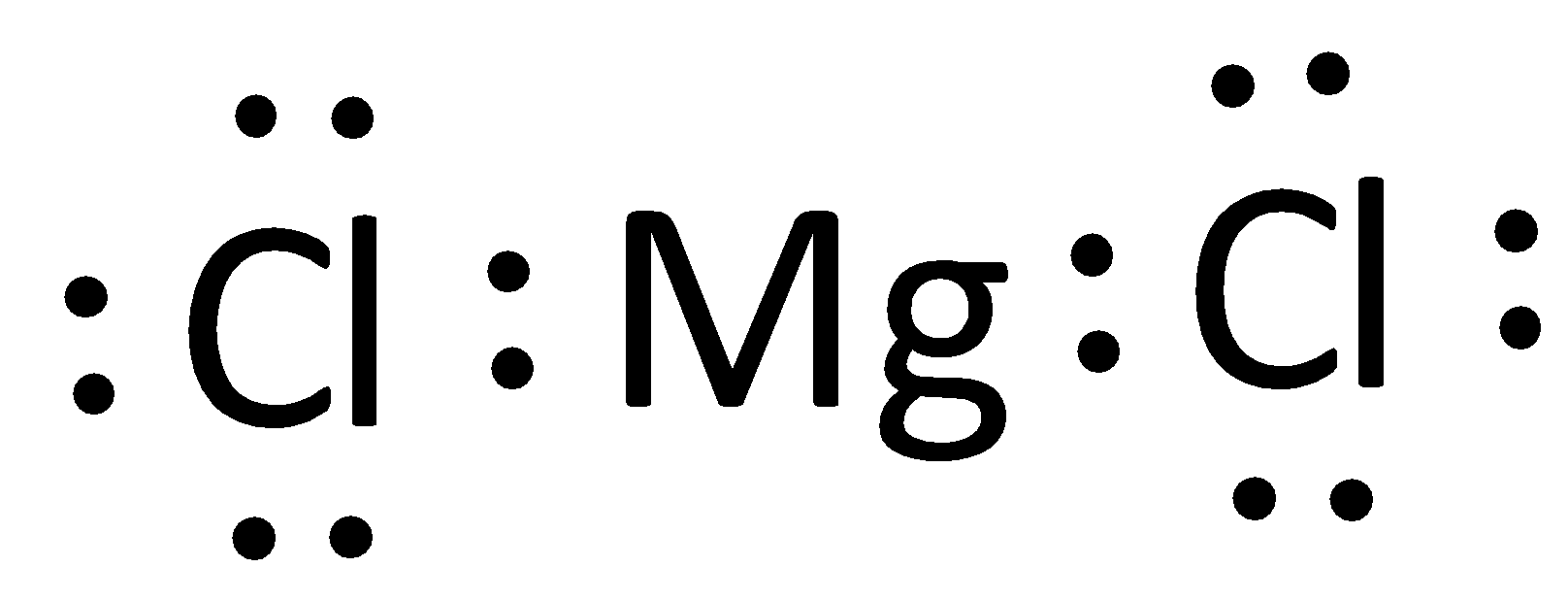
What is MgCl_2?
This type of bond involves the sharing of electrons between two nonmetals.
What is a covalent bond?
This biological process is used to create one of former chemistry teacher David Kriegshauser's favorite beverages.
What is fermentation?
When these conditions are met in a reversible reaction, the products of a reaction are favored
When is the Delta G negative and the Equilibrium is over 1?
This type of intermolecular force is experienced between two polar molecules.
What are diopole to diopole forces?
This is the Lewis dot diagram for SiCl_4.
What is:
This type of reaction occurs when a single compound breaks down into two or more simpler substances.
What is a decomposition reaction?
42.