What is another name for double-replacement reactions?
BONUS: Spell it correctly (+100 points)
Metathesis reaction
What is the formula for dilution?
Team 2 gets +300
M1V1 = M2V2
What does carbonic acid decompose into?
Carbon dioxide and water
In the electrolysis of water, which species is the reducing agent and the oxidizing agent?
X = n / n total, where X = mole fraction
True or False
When doing dilution problems, you have to convert mL to L
False.
What do metal hydroxides decompose into?
Metal hydroxides decompose into a metal oxide and water
How many moles of NaOH are used if the given concentration is 0.1 M and 150 mL?
BONUS: If you were given Molarity and moles, how would you find Volume? (+100)
M x V (in L)= n (moles)
(0.1 M)(0.150 L) = 0.015 moles NaOH
BONUS: n/M = L
What is the difference between monoprotic and diprotic acids?
Monoprotic acids have 1 H+ ion (proton) per molecule.
Diprotic acids have 2 H+ ions (protons) per molecule.
TWO Parts to earn full points:
i) Determine the salt product of a neutralization reaction between Hydrochloric acid and Sodium hydroxide (+150)
i) Is this salt soluble or insoluble? (+150)
i) The salt product is NaCl (Table salt)
ii) This is a soluble salt (according to solubility rules)
A student is performing a titration reaction between HCl and NaOH. 250 mL of 0.150 M NaOH solution is used to neutralize 100 mL HCl.
Calculate the unknown concentration of HCl.
M x V (in L) = (0.150 M)(0.250 L) = 0.0375 mol NaOH
MOLE RATIO (1:1 in this case):
(0.0375 mol NaOH/1 )(1 mol HCl/1 mol NaOH) = 0.0375 mol HCl
M (HCl) = n/V (in L) = (0.0375/0.1 L) =
0.375 M HCl!
We want to make sure all the carbonate decomposes, so we repeatedly heat and weigh it until we get a constant mass (indicates that all the carbonate decomposed)
In the activity series, hydrogen in an acid can be displaced by ___________ and hydrogen in water can be displaced by ________________
Team 1 gets -200 points
1) species above hydrogen (not below)
2) very reactive elements (e.g. Li, Na, K)
What volume should the student record based on the initial and final image of the buret?
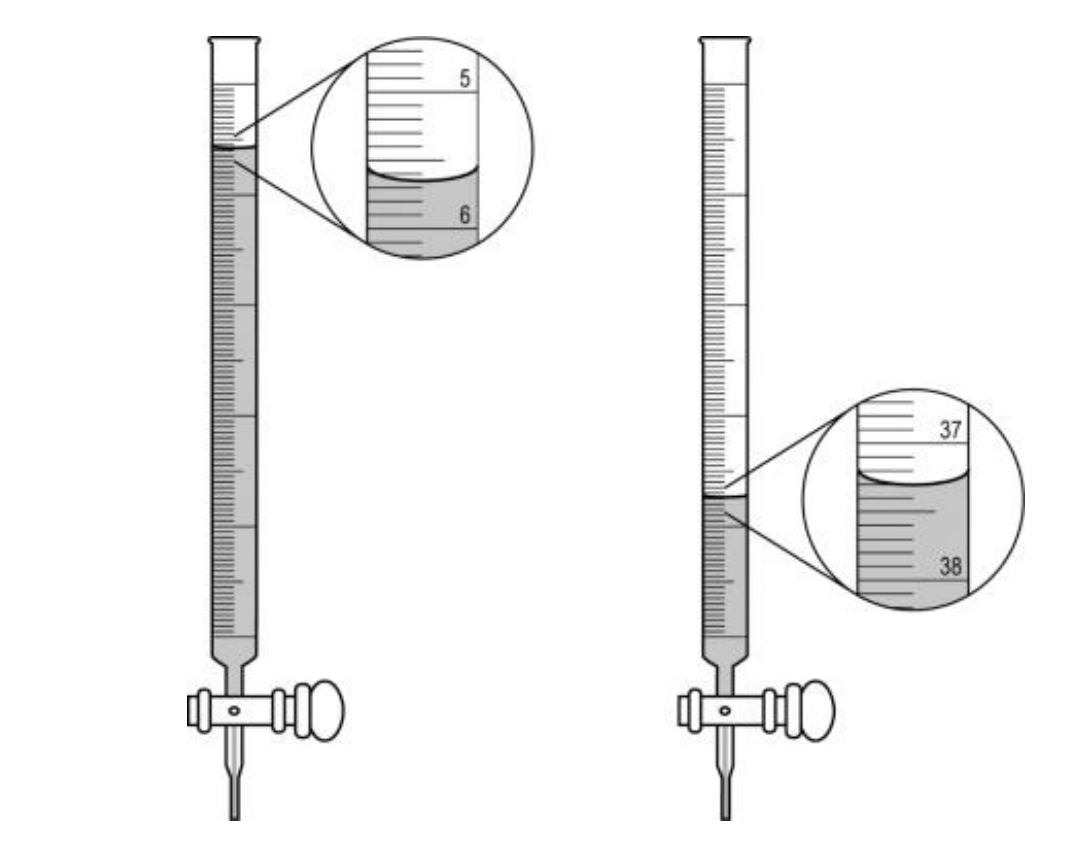
37.3 - 5.65 = 31.65 mL (simply 31 or 32 is not an acceptable answer)
Other acceptable answers:
31.6, 31.7
Which element is at the top of the activity series?
Team 3 gets +300 points
Potassium (K)