Which of the following best helps to account for the fact that the F- ion is smaller than the O2- ion?
A: F- has a larger nuclear mass than O2- has.
B: F- has a larger nuclear charge than O2- has.
C: F- has more electrons than O2- has.
D: F- is more electronegative than O2- is.
E: F- is more polarizable than O2- is.
B
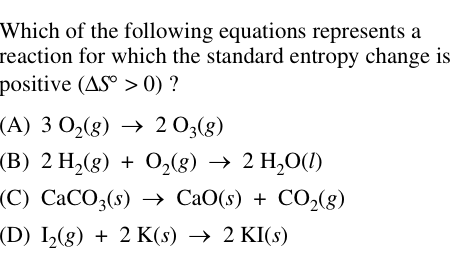
C
Which of the following methods is most appropriate to use to determine the number of different-colored components in a sample of black ink?
A: Distillation at atmospheric pressure
B: Elemental analysis to determine the mass ratio of C:H:N:O
C: Column chromatography using a nonpolar stationary phase and water as the mobile phase
D: Paper chromatography using different solvents with a range of polarities as the mobile phase
Answer D
Paper chromatography is appropriate to use to determine the number of components, because it can be used to separate very small quantities of a mixture into its components. Trying different solutions with a range of polarities as the mobile phase is helpful for identifying the solution that results in the greatest difference in retention factors among the components.
What is the solvent in an aqueous solution?
Water

Identify the Catalyst and it role
Ru, A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction.
Identify the Unknown Substance.
Al
Draw a diagram that best represents the structure of solid KF?


The two gas samples represented in the graph above are at the same temperature. Which of the following statements about the gases is correct?
A: The molecules of gas Z have a higher average kinetic energy than the molecules of gas X.B: There are fewer molecules in the sample of gas Z than in the sample of gas X.
C: Gas Z has a smaller molar mass than gas X.
D: Gas Z has a greater molar mass than gas X.
Answer C
Gases at the same temperature have the same average kinetic energy of their molecules. Since ��=12�X�X2=12�Z�Z2, the gas with the higher average molecular speed has the lower molar mass (�X/�Z=�Z2/�X2). The curve for gas Z shows that the molecules have a higher average speed than the molecules in gas X, so gas Z has a smaller molar mass.
A 0.100 M solution of NaOH is used to titrate an HCl solution of unknown concentration. To neutralize the solution, an average volume of the titrant was 38.2 mL. The starting volume of the HCl solution was 20 mL. What's the concentration of the HCl?
To find the concentration of the HCl solution, we can use the equation:
M1V1 = M2V2
Where:
M1 = concentration of NaOH solution
V1 = volume of NaOH solution used
M2 = concentration of HCl solution
V2 = volume of HCl solution used
Given:
M1 = 0.100 M
V1 = 38.2 mL
V2 = 20 mL
We can rearrange the equation to solve for M2:
M2 = (M1V1) / V2
M2 = (0.100 M * 38.2 mL) / 20 mL
M2 = 0.191 M
So, the concentration of the HCl solution is 0.191 M.
C12H2O11(aq)+H2O(l)→2C6H12O6(aq)
The chemical equation shown above represents the hydrolysis of sucrose. Under certain conditions, the rate is directly proportional to the concentration of sucrose. Which statement supports how a change in conditions can increase the rate of this reaction?
A: Increasing the amount of water in which the sugar is dissolved will increase the frequency of collisions between the sucrose molecules and the water molecules resulting in an increase in the rate of hydrolysis.
B: Decreasing the temperature will increase the frequency of the collisions between the sucrose molecules and the water molecules resulting in an increase in the rate of hydrolysis.
C: Increasing the concentration of sucrose will increase the rate of hydrolysis by increasing the frequency of the collisions between the sucrose and the water molecules.
D: Decreasing the concentration of sucrose will increase the rate of hydrolysis by increasing the frequency of the collisions between the sucrose and the water molecules.
Answer C
The rate changes proportionally to the change in the concentration of sucrose and the increase in the rate of hydrolysis can be explained by an increase in the frequency of the collisions between the reactant molecules.
The elements in which of the following have most nearly the same atomic radius?
A: Be, B, C, N
B: Ne, Ar, Kr, Xe
C: Mg, Ca, Sr, Ba
D: C, P, Se, I
E: Cr, Mn, Fe, Co
E

Which of the following can be inferred from the diagram above that shows the dependence of potential energy on the internuclear distance between two atoms?
A: The atoms form a bond with a bond length of 25pm.
B: The atoms form a bond with a bond length of 75pm.
C: The net force between the atoms is attractive at 25pm.
D: The net force between the atoms is attractive at 75pm.
Answer B
The minimum potential energy occurs at an internuclear distance of 75pm, which corresponds to the length of the stable bond that forms between the two atoms. If the atoms were any closer to each other, the net force would be repulsive. Likewise, if the atoms were farther from each other, the net force would be attractive. At 75pm, the net force between the two atoms is zero.

A solid compound of a group 1 (alkali) metal and a group 17 (halogen) element dissolves in water. The diagram above represents one type of solute particle present in the solution. Which of the following identifies the solute particle and best helps explain how the solute particle interacts with water molecules?
A: The particle is a negative ion, and the interactions are hydrogen bonds.
B: The particle is a negative ion, and the interactions are ion-dipole attractions.
C: The particle is a positive ion, and the interactions are ion-dipole attractions.
D: The particle is a positive ion, and the interactions are dipole-dipole attractions.
Answer C
The water molecules are all oriented the same way with respect to the solute particle, with the negative ends of the water molecule dipoles directed toward the solute particle. This can only be the case if the solute particle has a positive charge. The major attractive forces between the polar water molecules and the positive ion are characterized as ion-dipole attractions.
Which one of the following compounds is insoluble in water?
A) Ag2CO3
B) K2SO4
C) ZnS
D) Na2CO3
E) Fe(NO3)3
A and C
The mechanism for a chemical reaction is shown above. Which of the following statements about the overall reaction and rate laws of the elementary reactions is correct?
ResponsesA
The chemical equation for the overall reaction is 2 H2O2+I−→ 2 H2O+O2+I−, and the rate law for elementary step 2 is ����=�[H2O2][IO−].
B
The chemical equation for the overall reaction is H2O2+IO−→ H2O+O2+I−, and the rate law for elementary step 2 is ����=�[H2O2]2[IO−].
C
The chemical equation for the overall reaction is 2 H2O2→2 H2O+O2, and the rate law for elementary step 1 is ����=�[H2O2][I−].
D
The chemical equation for the overall reaction is 2 H2O2→2 H2O+O2, and the rate law for elementary step 1 is ����=�[H2O2]2.
Answer C
Correct. Once all the elementary steps are added and simplified, the resulting equation is 2 H2O2→2 H2O+O2. Because a rate law for an elementary step can be based on the stoichiometric coefficients of the reactants in that step, the rate law for elementary step 1 is ����=�[H2O2][I−], and the rate law for elementary step 2 is ����=�[H2O2][IO−].
A solution of methanol, CH3OH, in water is prepared by mixing together 128 g of methanol and 108 g of water. The mole fraction of methanol in the solution is closest to
A: 0.80
B: 0.60
C: 0.50
D: 0.40
E: 0.20
D
The elements C and Se have the same electronegativity value, 2.55. Which of the following claims about the compound that forms from C and Se is most likely to be true?
A: The carbon-to-selenium bond is unstable.
B: The carbon-to-selenium bond is nonpolar covalent.
C: The compound has the empirical formula CSe.
D: A molecule of the compound will have a partial negative charge on the carbon atom.
Answer B
The bonding in compounds between elements with the same (or only slightly different) electronegativity values will exhibit covalent bonding (that is, more or less equal sharing of electrons).
What volume of a 0.100 M HCl stock solution should be used to prepare 250.00mL of 0.0250 M HCl?
A: 1.00mL
B: 16.0mL
C: 62.5mL
D: 100mL
C
Combining aqueous solutions of BaI2 and K2SO4 affords a precipitate of BaSO4. Which ion(s) is/are spectator ions in the reaction?
A) Ba2+ only
B) K+ and I
C) SO42- and I
D) Ba2+ and SO4 2-
E) K+ only
B
The overall reaction represented above is proposed to take place through two elementary steps. Which of the following statements about the chemical equation for step 1 and the rate law for the overall reaction is correct?
ResponsesA
The chemical equation for step 1 is NO2(�)+F2(�)→NO2F(�)+F(�), and the rate law for the overall reaction is ����=�[NO2][F2].
B
The chemical equation for step 1 is NO2(�)+F(�)→NO2F(�)+F2(�), and the rate law for the overall reaction is ����=�[NO2][F].
C
The chemical equation for step 1 is 2NO2(�)+F2(�)→2NO2F(�), and the rate law for the overall reaction is ����=�[NO2][F].
D
The chemical equation for step 1 is 3NO2(�)+F2(�)+F(�)→3NO2F(�), and the rate law for the overall reaction is ����=�[NO2]2[F2].
Answer A
Correct. The chemical equations for the two elementary steps should add up to give the net equation for the overall reaction. Based on the chemical equations for step 2 and the overall reaction, the chemical equation for step 1 is NO2(�)+F2(�)→NO2F(�)+F(�). Because step 1 is the slow (rate-determining) step, the rate law for the overall reaction is set by the stoichiometry of step 1 and is equal to ����=�[NO2][F2].
The four species Cl-, Ar, K+, and Ca2+ have the same electron configuration, 1s22s22p63s2 3p6. Which of the following statements correctly identifies the species with the largest radius and provides an explanation based on Coulomb’s law?
A: Cl-, because its nuclear charge exerts the least attractive force on the electrons in the 3p sublevel.
B: Ar, because it is a neutral atom and the net force on the electrons in the 3p sublevel is zero.
C: K+, because the loss of an electron results in a smaller attractive force between the nucleus and the electrons in the 3p sublevel.
D: Ca2+, because the large positive charge on a cation causes increased repulsion among the electrons in the 3� sublevel.
A
According to Coulomb’s law, the radius of the atom or ion depends on the strength of attraction of the nuclear charge for the outer electrons. The greater the attraction, the smaller the radius. The attractive force exerted by the nucleus on an electron in the 3p sublevel increases with increasing charge of the nucleus (or with increasing number of protons). Cl- has the smallest number of protons among the species listed, and therefore the largest radius.
Two pure elements react to form a compound. One element is an alkali metal, X, and the other element is a halogen, Z. Which of the following is the most valid scientific claim that can be made about the compound?
A: It has the formula XZ2.
B: It does not dissolve in water.
C: It contains ionic bonds.
D: It contains covalent bonds.
Answer C
An alkali metal will readily lose an electron and a halogen will readily gain an electron to form an ionic compound with a 1:1 ratio of X+:Z- ions.

The graph above shows how a particular real gas deviates from ideal behavior at very high pressures. Based on this information, which of the following is most likely the gas and gives the reason based on kinetic molecular theory?
A: H2, because it has the smallest mass.
B: N2, because its molecules have a triple bond.
C: Ne, because it has a completely filled valence shell.
D: SO2, because it has the largest molecular volume.
Answer D
SO2 has the largest molecular volume. At very high pressures, the space occupied by the molecules is more significant and will result in the largest deviation from the volume predicted by the ideal gas law. According to kinetic molecular theory, ideal gas molecules have negligible volumes, so the larger its molecules are, the more the behavior of the gas will deviate from that of an ideal gas.
The balanced half-reaction in which dichromate ion is reduced to chromium metal is a __________ process. A) two-electron
B) six-electron
C) four-electron
D) twelve-electron
E) three-electron
D

The diagram above shows the reaction energy profiles for a reaction with and without a catalyst. Which of the following identifies the reaction energy profile for the catalyzed reaction, and why?
ResponsesA
Profile X, because a catalyst minimizes the number of elementary steps required for a reaction to proceed.
B
Profile X, because the activation energy for the reverse reaction is greater than for the forward reaction, which increases its rate.
C
Profile Y, because an increase in the number of transition states increases the rate of the reaction.
D
Profile Y, because it introduces a different reaction path that reduces the activation energy.
Answer D
Correct. A catalyst increases the rate of a chemical reaction by either increasing the number of effective collisions or, as in this case, providing a different reaction path with a lower activation energy.