Phosphorus has how many protons?
Fifteen.
What is the purpose of ICE table calculations?
To determine the equilibrium concentrations of all species in a system.
What is VSEPR the abbreviation for?
Valence shell electron-pair repulsion theory.
What is the species that sets the boundary of how much product may be formed.
The limiting reactant.
What is the value of the gas constant with correct units when the pressure is provided in atmospheres?
0.0821(L*atm)/(mol*K)
What is the name of the group of orbitals with the same principal quantum number.
An electron shell.
What is the molar ratio between sulfuric acid and sodium hydroxide?
The molar ratio is 2:1.
What is the name of this geometrical shape?

Trigonal pyramidial.
What is the name of the unit that describes the concentration of a solute in a solution in terms of the number of moles in a specified mass of solvent?
Molality.
A sample of 0.7 moles Br2 gas is held within a 4L vessel at 290oC, what is the pressure that it experiences in atmospheres?
8.1 atmospheres of pressure.
What is the electron configuration of sulfur?
1s22s22p63s23p4.
What is the name of the aqua-colored point and what is the classification of the acid in this system?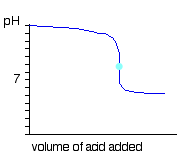
The end point; a weak acid.
Which atoms must a hydrogen atom be covalently bonded to for the hydrogen to participate in hydrogen bonding?
Fluorine, oxygen, or nitrogen are electronegative enough to allow for hydrogen atoms to hydrogen bond.
[Remember: FON]
What is the number of moles in 23mL of a 0.92M HCl solution?
0.02116 moles.
Which flask has the greatest number of molecules?
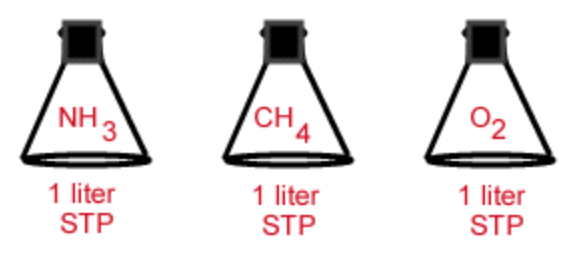
All have the same number of molecules.
What are the masses of the two comparable subatomic atomic particles and their identities?
Proton and neutron, both having a mass of ~1 amu.
[The proton has a mass of 1.007 amu and the neutron has a mass of 1.009 amu.]
What is the pH of a 0.62 KOH solution?
pH = 13.8.
The name of the intermolecular attraction shown below is?
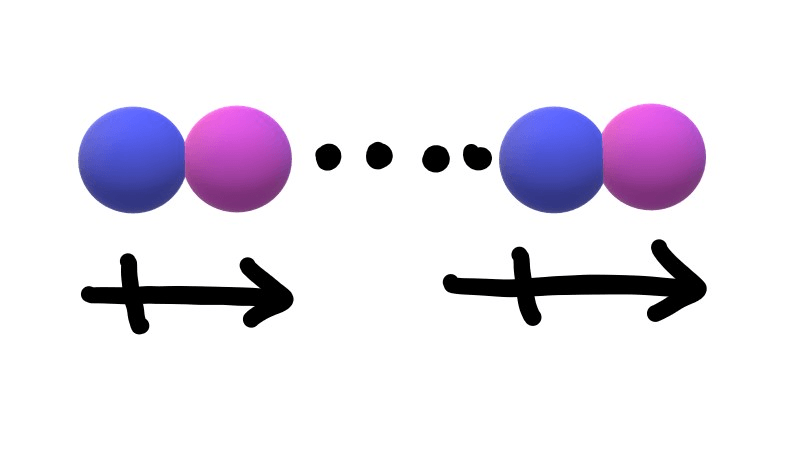
Dipole-dipole attraction.
What is the concentration of a 30g sample of Ni(NO3)2 dissolved in 250mL of water?
0.657M.
A mixture of two gases, CH4 and C2H2, has the properties listed below. What is the PC2H2 for this mixture?
PT=920 torr
ΧCH4=0.43
PC2H2 = 520 torr
Which of the following elements has the largest first ionization energy?
Helium, lithium, or oxygen?
Helium, which has the highest of any element's ionization energy.
Hypobromous acid has a Ka of 2.818x10-9 and acetic acid has a Ka of 1.75x10-5. Which acid will produce the higher strength conjugate base?
Hypobromous acid.
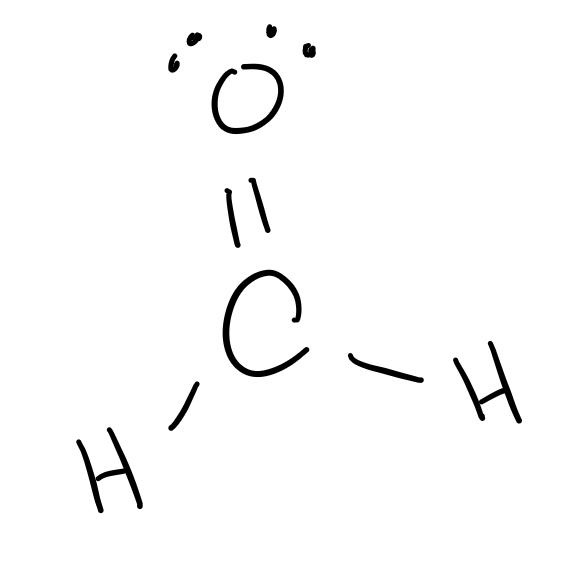
Draw the structure of COH2 and label the hybridization about the carbon atom.
[Hint: hybridization options are sp, sp2, or sp3.]
Hybridization: sp2.
Three identical balloons are filled with equal volumes of three separate gases (H2O, O3, and Xe). All of the balloons are at 27oC and 1.5 atm pressure. Which balloon has the greatest kinetic energy?
All have equal kinetic energy because temperature is held the same for all.
What is the molar mass of a 0.5g sample of a mystery gas in a 2L vessel at 4.2 atm and 150oC? What is its identity?
H2 gas; molar mass of 2.07g/mol.